Question: Exercise 1.4 Catalytic Packed Bed Reactor This example solves the mole balance in a packed bed catalytic reactor using the catalyst weight as the independent
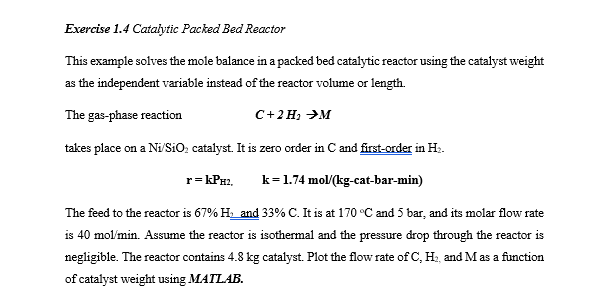
Exercise 1.4 Catalytic Packed Bed Reactor This example solves the mole balance in a packed bed catalytic reactor using the catalyst weight as the independent variable instead of the reactor volume or length. The gas-phase reaction C+2H M takes place on a Ni/SiO catalyst. It is zero order in C and first-order in Hz. r=kPH2. k=1.74 mol (kg-cat-bar-min) The feed to the reactor is 67% Hand 33% C. It is at 170C and 5 bar, and its molar flow rate is 40 mol/min. Assume the reactor is isothermal and the pressure drop through the reactor is negligible. The reactor contains 4.8 kg catalyst. Plot the flow rate of C, H and Mas a function of catalyst weight using MATLAB. Exercise 1.4 Catalytic Packed Bed Reactor This example solves the mole balance in a packed bed catalytic reactor using the catalyst weight as the independent variable instead of the reactor volume or length. The gas-phase reaction C+2H M takes place on a Ni/SiO catalyst. It is zero order in C and first-order in Hz. r=kPH2. k=1.74 mol (kg-cat-bar-min) The feed to the reactor is 67% Hand 33% C. It is at 170C and 5 bar, and its molar flow rate is 40 mol/min. Assume the reactor is isothermal and the pressure drop through the reactor is negligible. The reactor contains 4.8 kg catalyst. Plot the flow rate of C, H and Mas a function of catalyst weight using MATLAB
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


