Question: Example 6.7-28 Determine the equilibrium conversion for the isomerization reaction of methylcyclopentane (CH:CzH9) to cyclohexane (C.H.2) at 298K. Gibbs energies of formation are given at
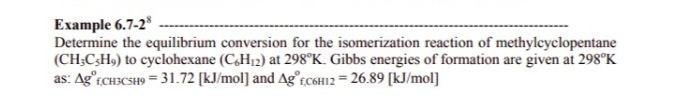
Example 6.7-28 Determine the equilibrium conversion for the isomerization reaction of methylcyclopentane (CH:CzH9) to cyclohexane (C.H.2) at 298K. Gibbs energies of formation are given at 298K as: AgrChaCsH9 = 31.72 [kJ/mol) and Ag fC6H12 = 26.89 [kJ/mol] f.CH3C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


