Question: Example: An aqueous solution containing 40 mole percent acetone is to be flash distilled at 760mmHg pressure. Calculate the compositions of top and bottom products
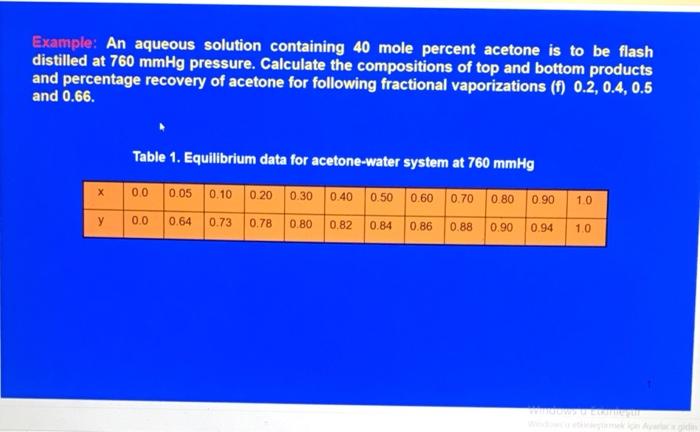
Example: An aqueous solution containing 40 mole percent acetone is to be flash distilled at 760mmHg pressure. Calculate the compositions of top and bottom products and percentage recovery of acetone for following fractional vaporizations (f) 0.2,0.4,0.5 and 0.66. Table 1. Equilibrium data for acetone-water system at 760mmHg Example: An aqueous solution containing 40 mole percent acetone is to be flash distilled at 760mmHg pressure. Calculate the compositions of top and bottom products and percentage recovery of acetone for following fractional vaporizations (f) 0.2,0.4,0.5 and 0.66. Table 1. Equilibrium data for acetone-water system at 760mmHg
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


