Question: Example: For the following reaction, you know that the initial concentration of A was 1.50M and B was 0 . When equilibrium was reached, the
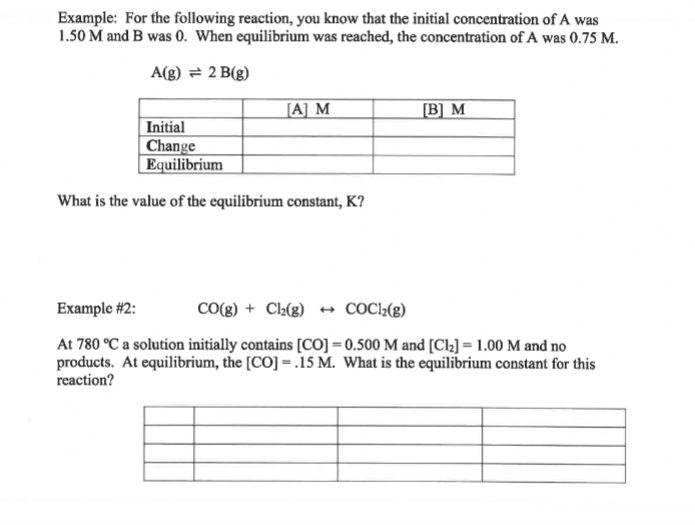
Example: For the following reaction, you know that the initial concentration of A was 1.50M and B was 0 . When equilibrium was reached, the concentration of A was 0.75M. A(g)2B(g) What is the value of the equilibrium constant, K ? Example \#2: CO(g)+Cl2(g)COCl2(g) At 780C a solution initially contains [CO]=0.500M and [Cl2]=1.00M and no products. At equilibrium, the [CO]=.15M. What is the equilibrium constant for this reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


