Question: Example Problem The hypothetical ion Adamantium ( Ad ) carries a single positive charge charge ( ( mathrm { Ad } ^ {
Example Problem
The hypothetical ion Adamantium Ad carries a single positive charge charge mathrmAd Its extracellular concentration is mM whereas the internal concentration is mM
a First, consider the effect of concentration gradient. Does concentration gradient create a tendency to push Adamantium outside, or pull them inside? Briefly explainillustrate
b For the intracellular resting potential of mV does the Electric field across the membrane have the tendency to push Adamantium outside, or pull them inside? Briefly explain.
c Calculate the Nernst potential for Adamantium at K Remember that the Nernst potential is drawn with opposite polarity to the cell membrane potential; you should obtain a positive value
d Draw the circuit diagram for Adamantium. The diagram should include a symbolic cell capacitance, the Nernst potential for this ion show polarity and value and the conductance path for the ion.
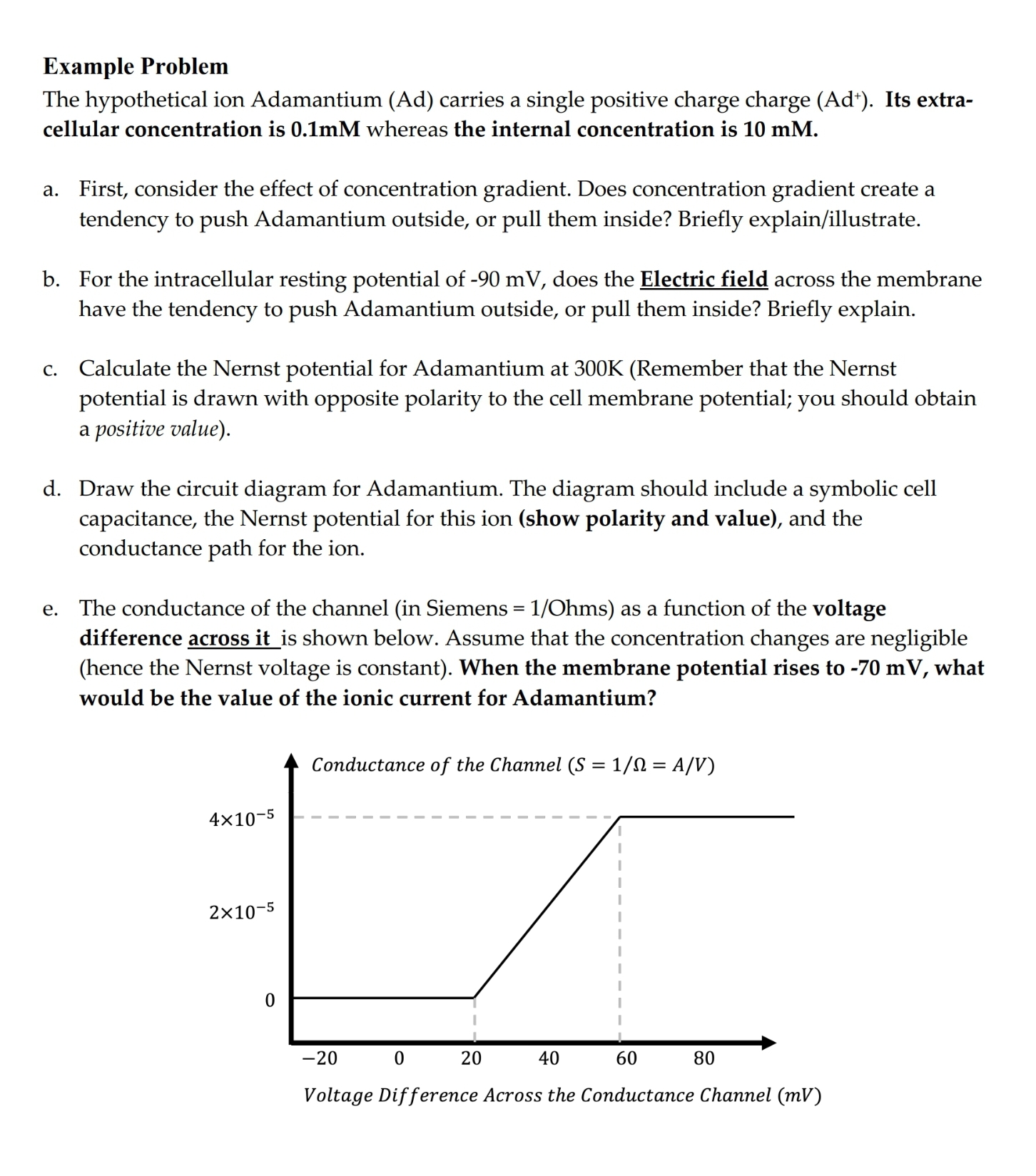
e The conductance of the channel in Siemens mathrmOhms as a function of the voltage difference across it is shown below. Assume that the concentration changes are negligible hence the Nernst voltage is constant When the membrane potential rises to mV what would be the value of the ionic current for Adamantium?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


