Question: Please answer all the above questions correctly providing necessary formulae, equations, expressions, diagrams, charts, graphs, plots and tables. 3. The catalytic cracking of gas oil
Please answer all the above questions correctly providing necessary formulae, equations, expressions, diagrams, charts, graphs, plots and tables.
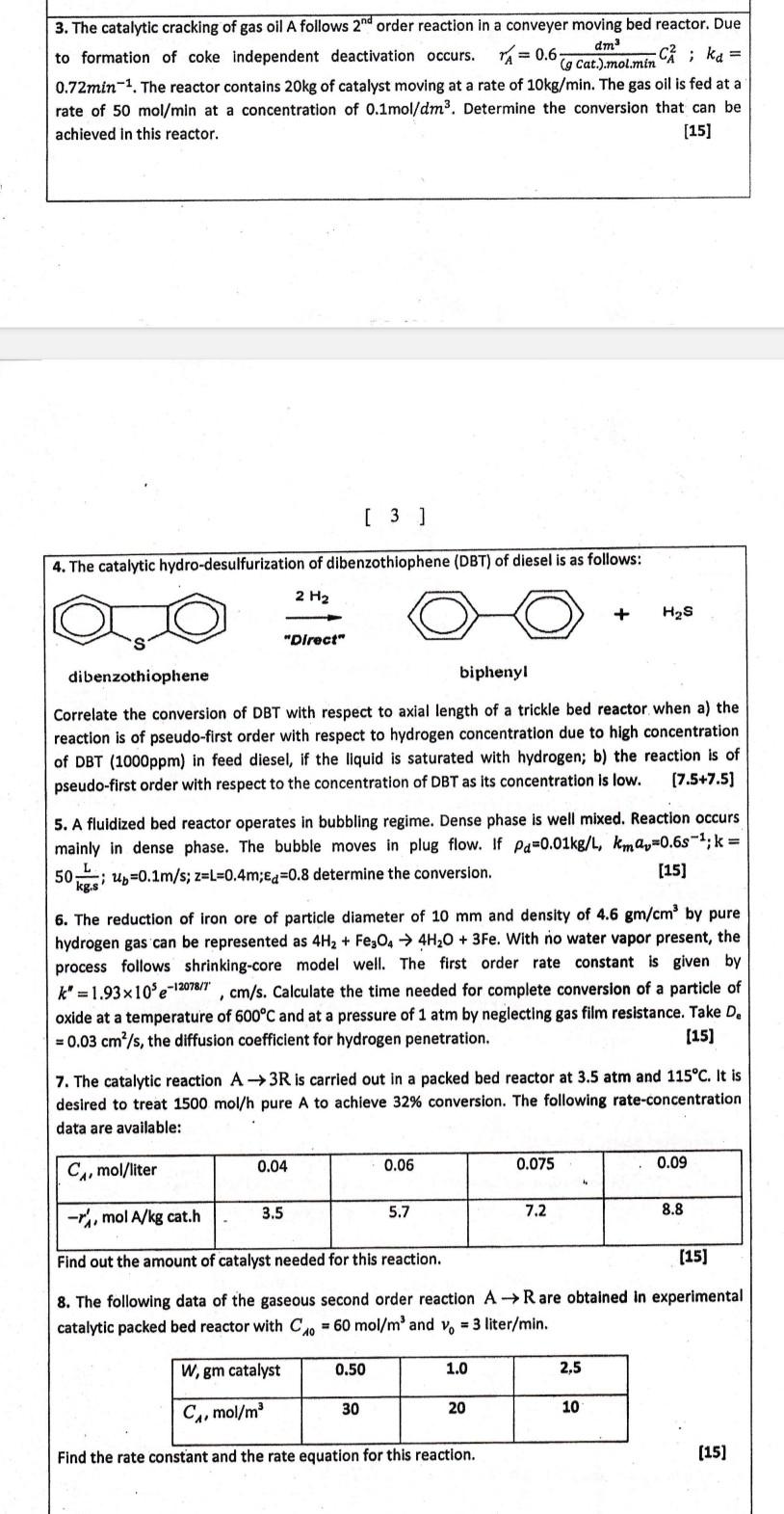
3. The catalytic cracking of gas oil A follows 2nd order reaction in a conveyer moving bed reactor. Due to formation of coke independent deactivation occurs. rA=0.6(gCat.)molmindm3CA2;kd= 0.72min1. The reactor contains 20kg of catalyst moving at a rate of 10kg/min. The gas oil is fed at a rate of 50mol/min at a concentration of 0.1mol/dm3. Determine the conversion that can be achieved in this reactor. [15] 4. The catalytic hydro-desulfurization of dibenzothiophene (DBT) of diesel is as follows: Correlate the conversion of DBT with respect to axial length of a trickle bed reactor when a) the reaction is of pseudo-first order with respect to hydrogen concentration due to high concentration of DBT (1000ppm) in feed diesel, if the liquid is saturated with hydrogen; b) the reaction is of pseudo-first order with respect to the concentration of DBT as its concentration is low. [7.5+7.5] 5. A fluidized bed reactor operates in bubbling regime. Dense phase is well mixed. Reaction occurs mainly in dense phase. The bubble moves in plug flow. If d=0.01kg/L,kmav=0.6s1;k= 50kg.sL;ub=0.1m/s;z=L=0.4m;d=0.8 determine the conversion. [15] 6. The reduction of iron ore of particle diameter of 10mm and density of 4.6gm/cm3 by pure hydrogen gas can be represented as 4H2+Fe3O44H2O+3Fe. With no water vapor present, the process follows shrinking-core model well. The first order rate constant is given by k=1.93105e12078/7,cm/s. Calculate the time needed for complete conversion of a particle of oxide at a temperature of 600C and at a pressure of 1atm by neglecting gas film resistance. Take De =0.03cm2/s, the diffusion coefficient for hydrogen penetration. [15] 7. The catalytic reaction A3R is carried out in a packed bed reactor at 3.5atm and 115C. It is desired to treat 1500mol/h pure A to achieve 32% conversion. The following rate-concentration data are available: 8. The following data of the gaseous second order reaction AR are obtained in experimental catalytic packed bed reactor with C10=60mol/m3 and v0=3liter/min. Find the rate constant and the rate equation for this reaction. [15] 3. The catalytic cracking of gas oil A follows 2nd order reaction in a conveyer moving bed reactor. Due to formation of coke independent deactivation occurs. rA=0.6(gCat.)molmindm3CA2;kd= 0.72min1. The reactor contains 20kg of catalyst moving at a rate of 10kg/min. The gas oil is fed at a rate of 50mol/min at a concentration of 0.1mol/dm3. Determine the conversion that can be achieved in this reactor. [15] 4. The catalytic hydro-desulfurization of dibenzothiophene (DBT) of diesel is as follows: Correlate the conversion of DBT with respect to axial length of a trickle bed reactor when a) the reaction is of pseudo-first order with respect to hydrogen concentration due to high concentration of DBT (1000ppm) in feed diesel, if the liquid is saturated with hydrogen; b) the reaction is of pseudo-first order with respect to the concentration of DBT as its concentration is low. [7.5+7.5] 5. A fluidized bed reactor operates in bubbling regime. Dense phase is well mixed. Reaction occurs mainly in dense phase. The bubble moves in plug flow. If d=0.01kg/L,kmav=0.6s1;k= 50kg.sL;ub=0.1m/s;z=L=0.4m;d=0.8 determine the conversion. [15] 6. The reduction of iron ore of particle diameter of 10mm and density of 4.6gm/cm3 by pure hydrogen gas can be represented as 4H2+Fe3O44H2O+3Fe. With no water vapor present, the process follows shrinking-core model well. The first order rate constant is given by k=1.93105e12078/7,cm/s. Calculate the time needed for complete conversion of a particle of oxide at a temperature of 600C and at a pressure of 1atm by neglecting gas film resistance. Take De =0.03cm2/s, the diffusion coefficient for hydrogen penetration. [15] 7. The catalytic reaction A3R is carried out in a packed bed reactor at 3.5atm and 115C. It is desired to treat 1500mol/h pure A to achieve 32% conversion. The following rate-concentration data are available: 8. The following data of the gaseous second order reaction AR are obtained in experimental catalytic packed bed reactor with C10=60mol/m3 and v0=3liter/min. Find the rate constant and the rate equation for this reaction. [15]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


