Question: Exercise 3.7: Creating a second phase with reaction Consider the gas-phase reaction A + BC (3.82) Product C has a fairly low vapor pressure, so
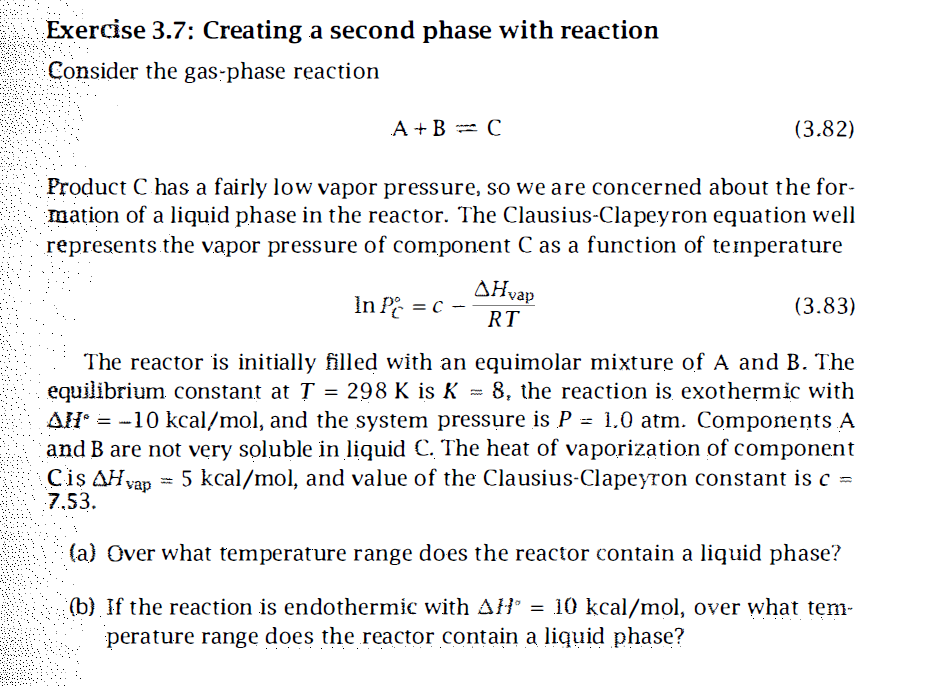
Exercise 3.7: Creating a second phase with reaction Consider the gas-phase reaction A + BC (3.82) Product C has a fairly low vapor pressure, so we are concerned about the for- mation of a liquid phase in the reactor. The Clausius-Clapeyron equation well represents the vapor pressure of component C as a function of temperature AHvap In PC = C - (3.83) RT = The reactor is initially filled with an equimolar mixture of A and B. The equilibrium constant at T = 298 K is K = 8, the reaction is exothermic with Al' = -10 kcal/mol, and the system pressure is P = 1,0 atm. Components A and B are not very soluble in liquid C. The heat of vaporization of component = 5 kcal/mol, and value of the Clausius-Clapeyron constant is c = 7.53. Cis AH yap (a) Over what temperature range does the reactor contain a liquid phase? (b) If the reaction is endothermic with AH = 10 kcal/mol, over what tem- perature range does the reactor contain a liquid phase
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


