Question: Exercise 6.101 an Review Const Part A MISSED THIS? Read Section 6.9. You can click on the Review link to access the section in your
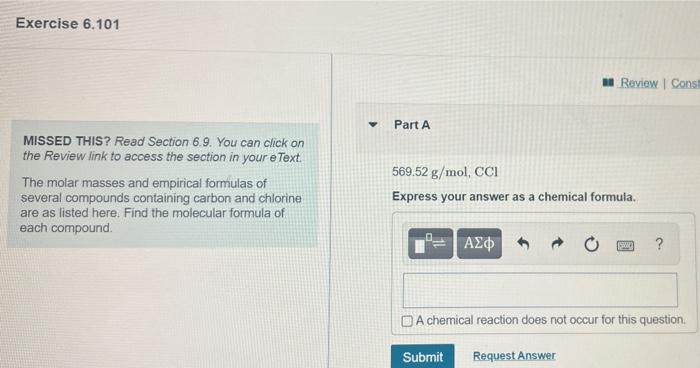
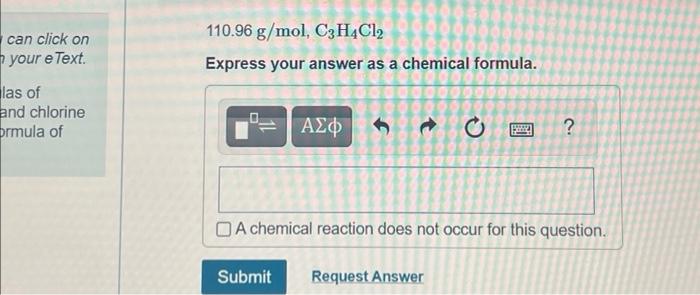
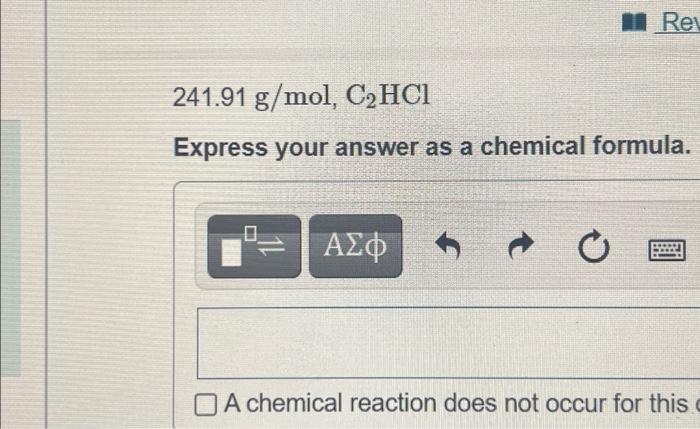
Exercise 6.101 an Review Const Part A MISSED THIS? Read Section 6.9. You can click on the Review link to access the section in your e Text The molar masses and empirical formulas of several compounds containing carbon and chlorine are as listed here. Find the molecular formula of each compound 569.52 g/mol, CCI Express your answer as a chemical formula. ? A chemical reaction does not occur for this question Submit Request Answer can click on your e Text 110.96 g/mol, C3H4Cl2 Express your answer as a chemical formula. las of and chlorine prmula of t o 2 ? A chemical reaction does not occur for this question, Submit Request Answer Rei 241.91 g/mol, C2HCI Express your answer as a chemical formula. O A chemical reaction does not occur for this
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


