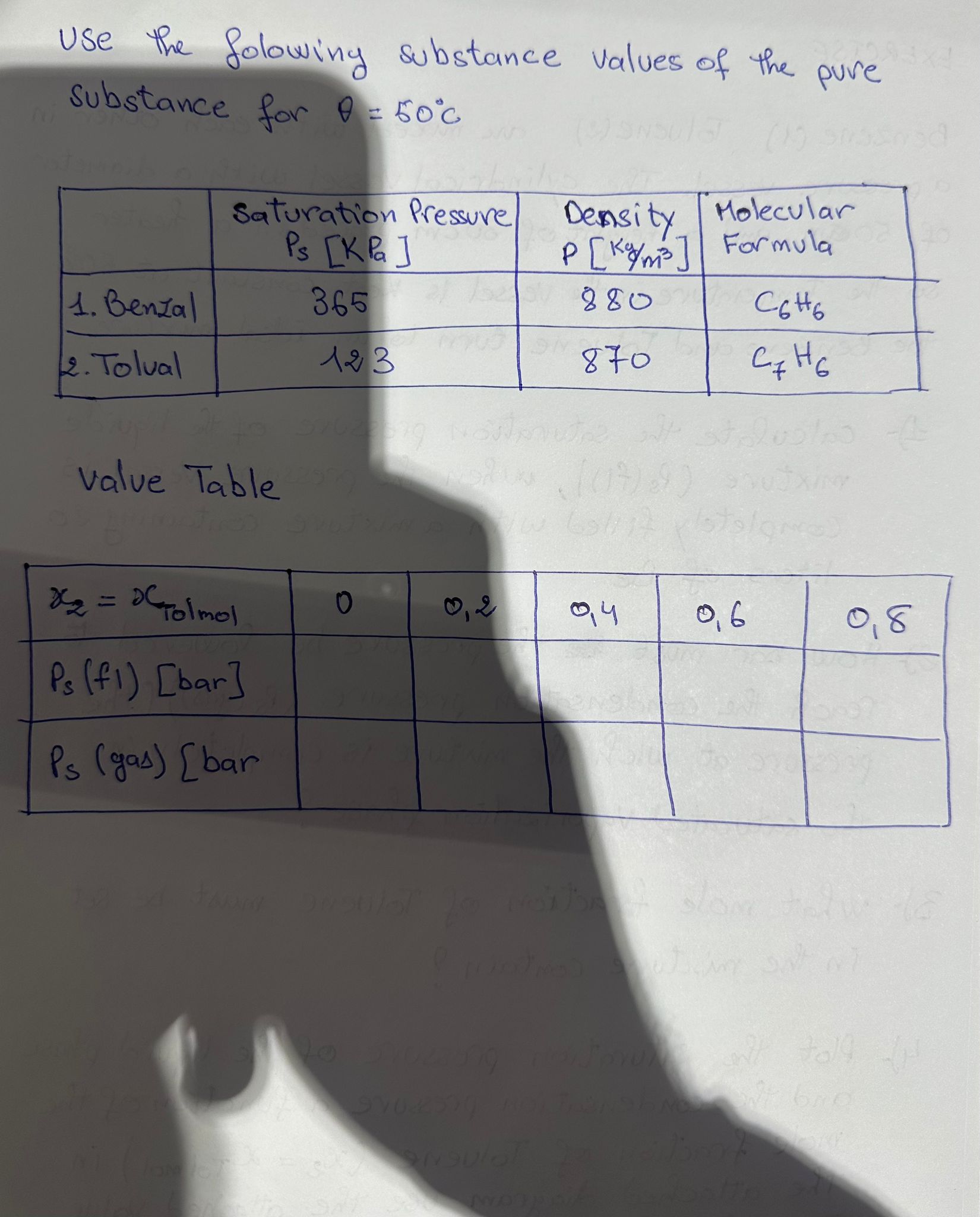
Question: EXERCISE Benzene ( 1 ) Toluene ( 2 ) are mixed with each other in a pressure vessel. The cylindrical vessel with a diameter of
EXERCISE
Benzene Toluene are mixed with each other in
a pressure vessel. The cylindrical vessel with a diameter
of and a height of placed in a heater
So the temperature in the vessel is kept constant at
the benzene and Toluene turn to an ideal mixture.
Calculate the saturation pressure of the liquide
mixture Psfl when the pressure vessel is
completely filled with a mixture containing
liters of Be
How bar must the pressure be lowered to
reach the condensation pressure Psgasthe
pressure at wich the mixture is completely in
the saturated vaporization phase?
What mole fraction of Toluene must be set
in the mixture contain?
Plot the saturation pressure of the liquid phase
and the condensation pressure a function of the
mole fraction of Tolvene in
The attached diagram use the attached value
Table for this purpose Use the folowing substance values of the pure
substance for
value Table
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


