Question: Exercise - Lever rule = > b - m a d - a L b A mixture of A 3 0 % + B 7
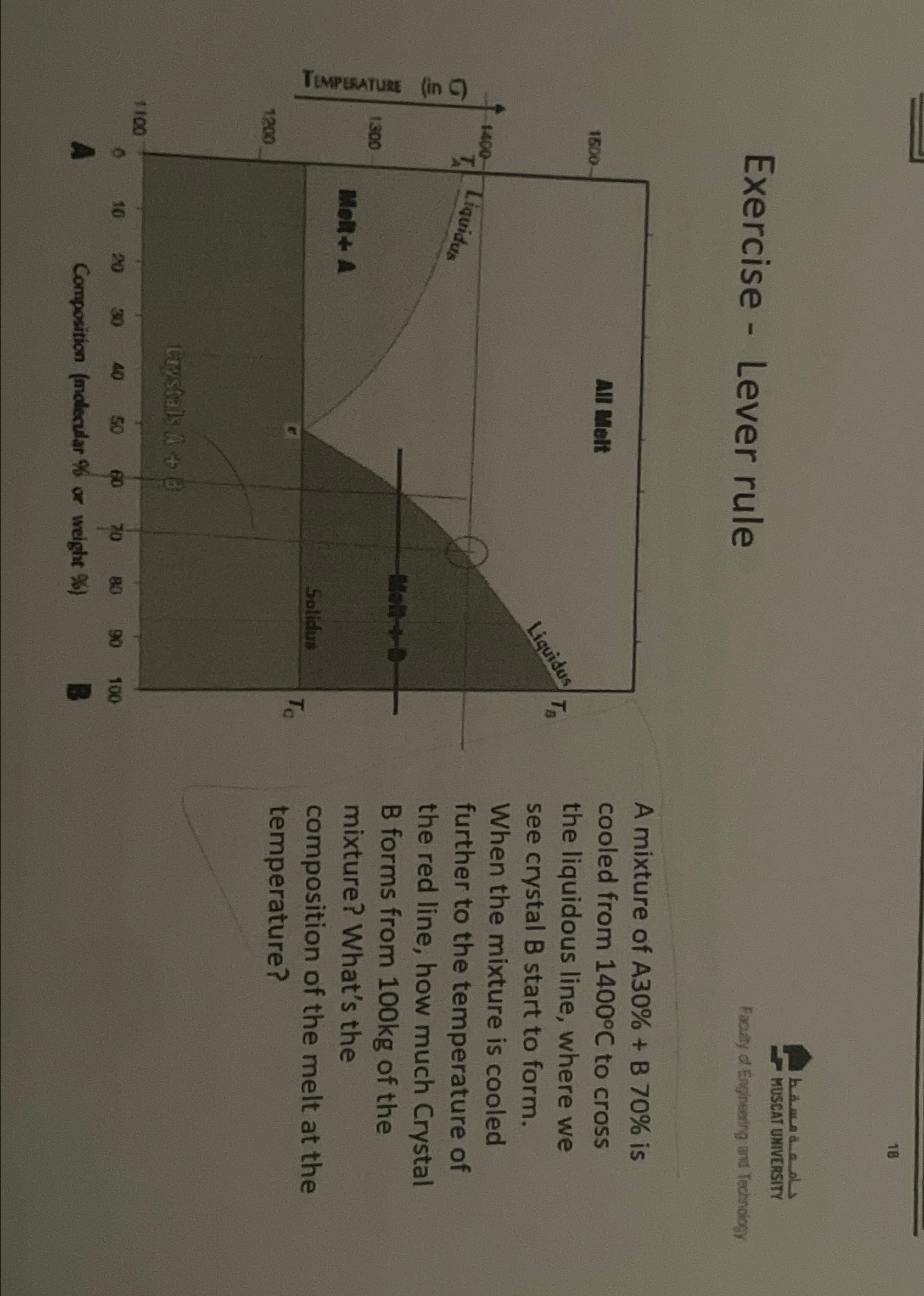
Exercise Lever rule
A mixture of is cooled from to cross the liquidous line, where we see crystal B start to form. When the mixture is cooled further to the temperature of the red line, how much Crystal B forms from of the mixture? What's the composition of the melt at the temperature?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


