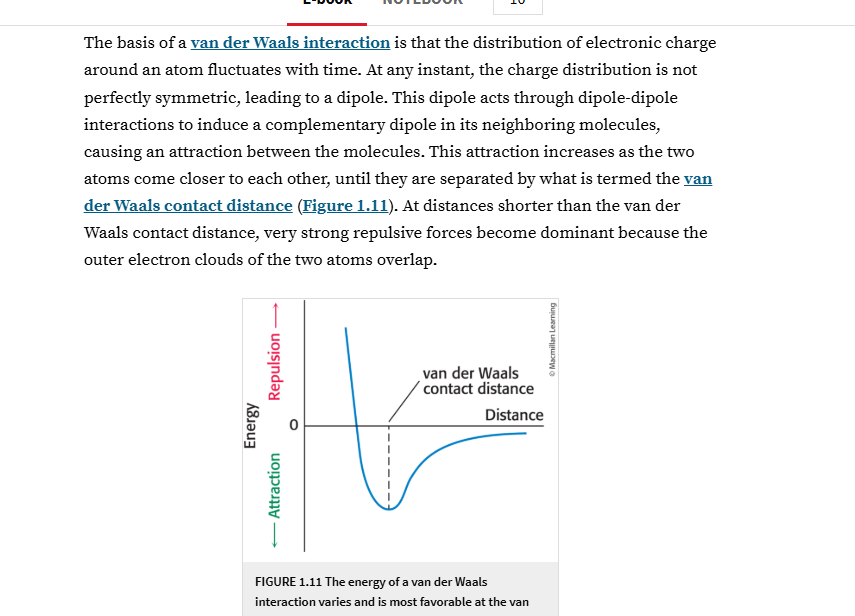
Question: explain in simple words a al ad UT, ee a ow The basis of a van der Waals interaction is that the distribution of electronic
explain in simple words
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


