Question: Explain neatly with explanation for each! 3. Structure and Bonding Consider the typical properties of each of the following substances (A to E): Substance Solubility
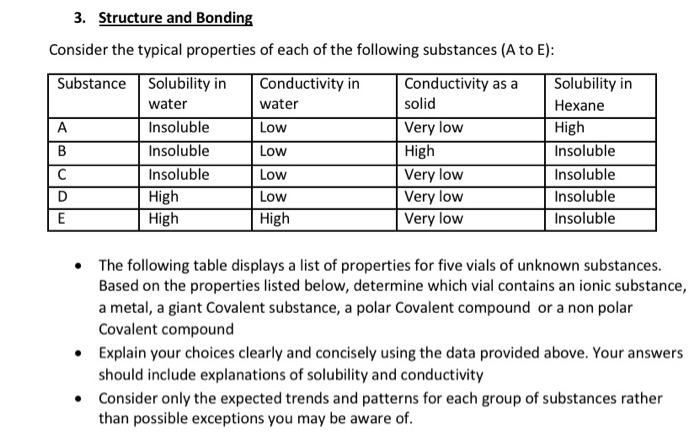
3. Structure and Bonding Consider the typical properties of each of the following substances (A to E): Substance Solubility in Conductivity in Conductivity as a Solubility in water water solid Hexane A Insoluble Low Very low High B Insoluble Low High Insoluble Insoluble Low Very low Insoluble D High Low Very low Insoluble E High High Very low Insoluble The following table displays a list of properties for five vials of unknown substances. Based on the properties listed below, determine which vial contains an ionic substance, a metal, a giant Covalent substance, a polar Covalent compound or a non polar Covalent compound Explain your choices clearly and concisely using the data provided above. Your answers should include explanations of solubility and conductivity Consider only the expected trends and patterns for each group of substances rather than possible exceptions you may be aware of
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


