Question: explain step by step with basics Methane and steam are fed to a reactor in molar ratio 1: 2. The following reactions take place, CH4


explain step by step with basics
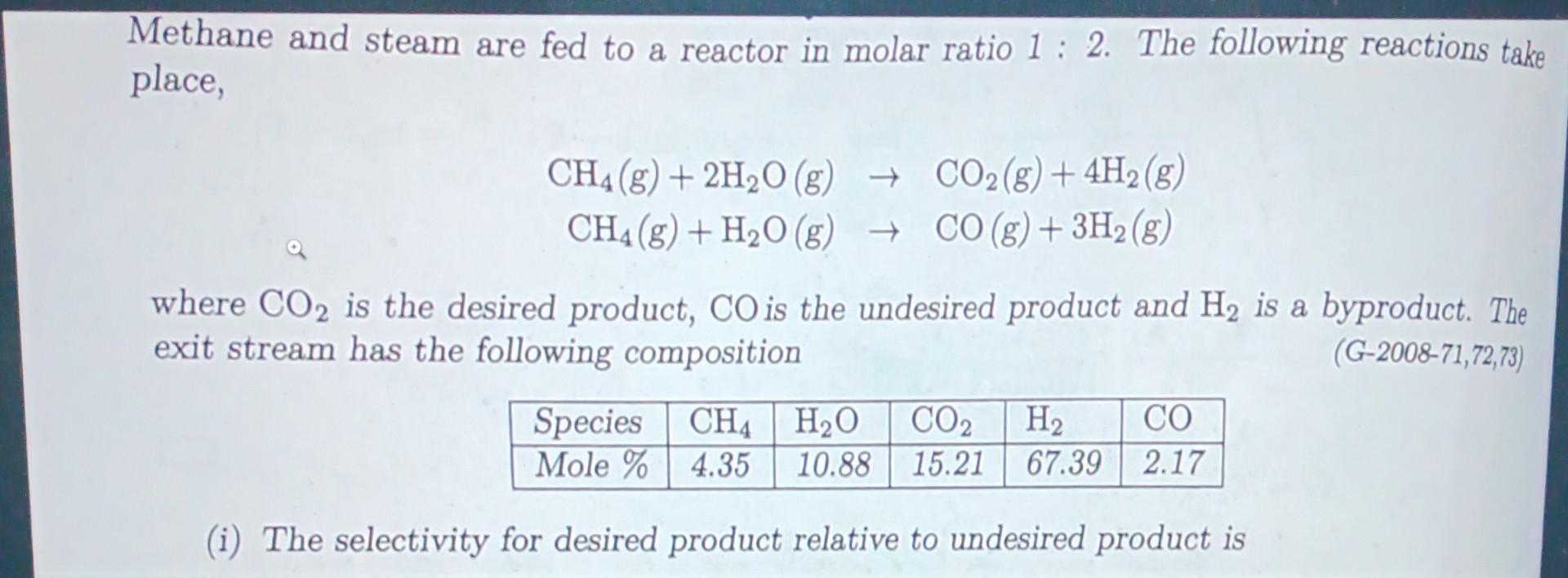
Methane and steam are fed to a reactor in molar ratio 1: 2. The following reactions take place, CH4 (8) + 2H2O(g) + CO2(g) + 4H2 (8) CH4 (8) + H2O(g) + CO(g) + 3H2 (8) where CO2 is the desired product, CO is the undesired product and H2 is a byproduct. The exit stream has the following composition (G-2008-71, 72,73) Species CH4 H2O CO2 H2 CO Mole % 4.35 10.88 15.21 67.39 2.17 (i) The selectivity for desired product relative to undesired product is (ii) The fractional yield of CO2 is (where fractional yield is defined as the ratio of moles of the desired product formed to the moles that would have been formed if there were no side reactions and the limiting reactant had reacted completely) (iii) The fractional conversion of methane is
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


