Question: explain steps and the equation used At 448C, the equilibrium constant Kc for the reaction H2(g)+I2(g)2HI(g) is 50.5. Predict in which direction the reaction proceeds
explain steps and the equation used 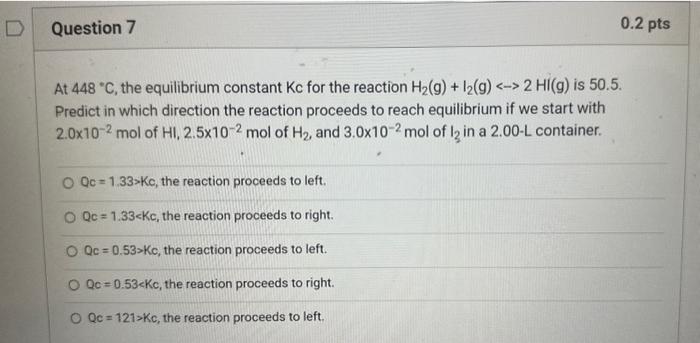

At 448C, the equilibrium constant Kc for the reaction H2(g)+I2(g)2HI(g) is 50.5. Predict in which direction the reaction proceeds to reach equilibrium if we start with 2.0102mol of HI,2.5102mol of H2, and 3.0102mol2 of I2 in a 2.00 - L container. Qc=1.33Kc, the reaction proceeds to left. Qc=1.33
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


