Question: Explain the question and answer 1. Write equations for the reaction of the buffer CH3COOH/CH3COO-buffer reacting with HCl and NaOH. Reaction with HCl : Dissociate
Explain the question and answer
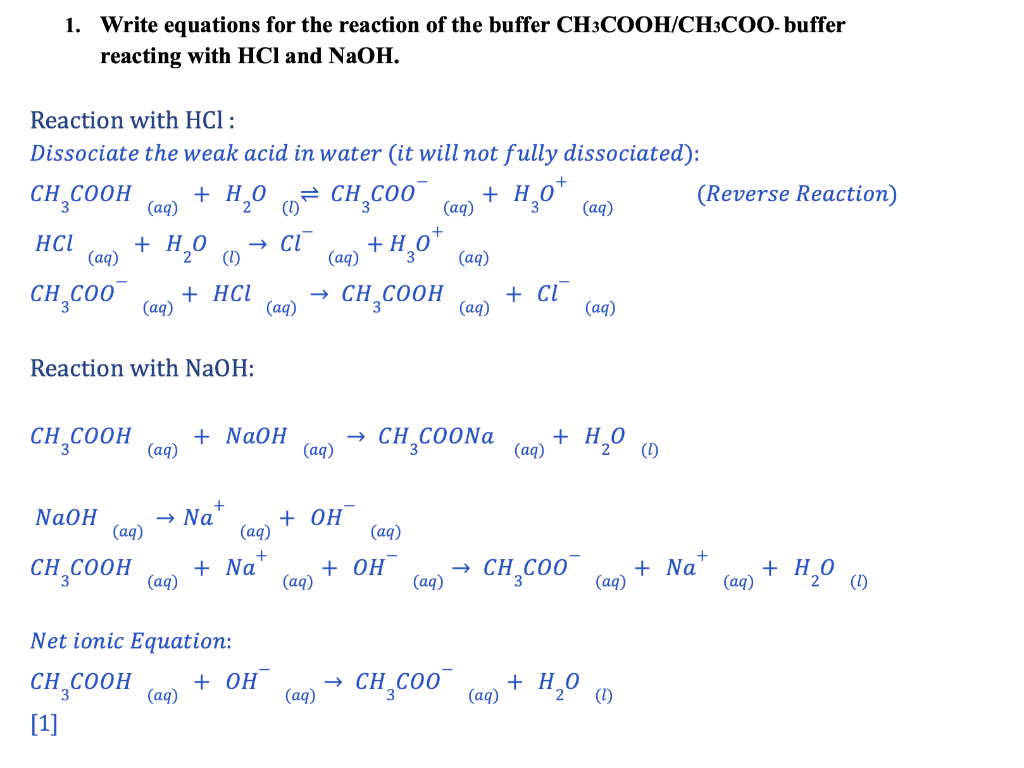
1. Write equations for the reaction of the buffer CH3COOH/CH3COO-buffer reacting with HCl and NaOH. Reaction with HCl : Dissociate the weak acid in water (it will not fully dissociated): CH3COOH(aq)+H2O(l)CH3COO(aq)+H3O+HCl(aq)+H2O(l)Cl(aq)+H3O(aq)+CH3COO(aq)+HCl(aq)CH3COOH(aq)+Cl (Reverse Reaction Reaction with NaOH : CH3COOH(aq)+NaOH(aq)CH3COONa(aq)+H2O NaOH(aq)Na(aq)++OHCH3COOH(aq)+Na(aq)++OH(aq)CH3COO(aq)+Na(aq)++H2O Net ionic Equation: CH3COOH(aq)+OH(aq)CH3COO(aq)+H2O
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


