Question: Explain why low temperatures are used in the reaction. - include the possible products that can form if the high temperature was used. How is
Explain why low temperatures are used in the reaction.
- include the possible products that can form if the high temperature was used.
How is deprotonation of the carbonate anion prevented? What reaction conditions were used to prevent this?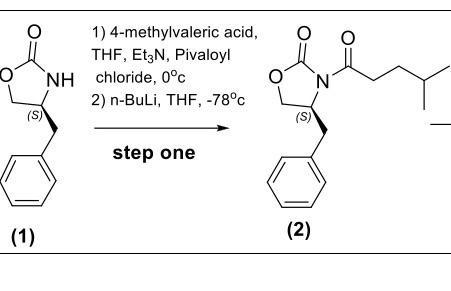
O O (S) (1) 1) 4-methylvaleric acid, THF, Et3N, Pivaloyl NH chloride, 0c 2) n-BuLi, THF, -78c step one (S) (2) N
Step by Step Solution
3.30 Rating (153 Votes )
There are 3 Steps involved in it
The low temperatures used in the reaction are likely to prevent unwanted side reactions and to promo... View full answer

Get step-by-step solutions from verified subject matter experts


