Question: For oxygen gas, the van der Waals equation of state achieves its best fit for a = 0.14 N m4/m012 and b 3.2 10
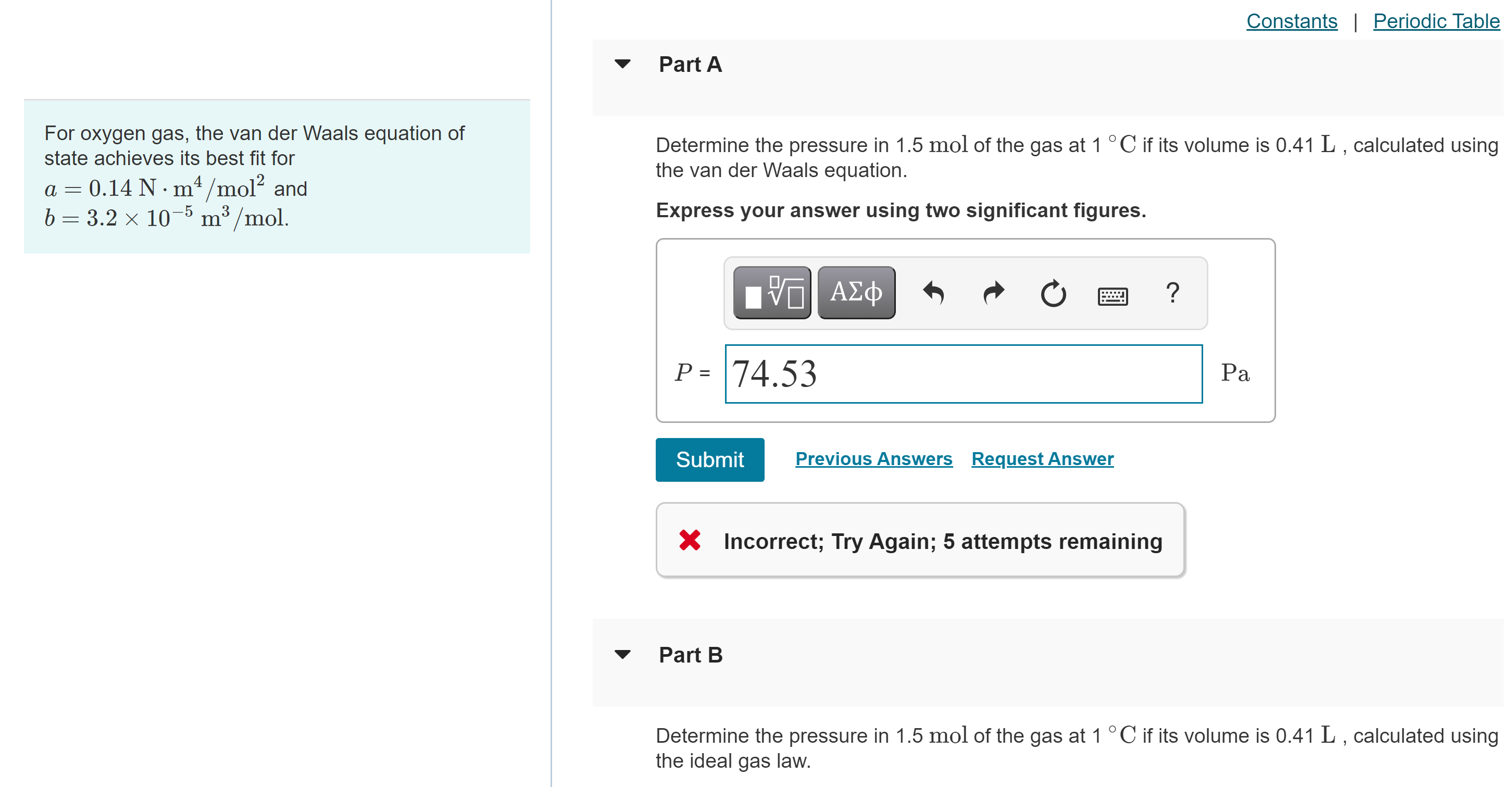
For oxygen gas, the van der Waals equation of state achieves its best fit for a = 0.14 N m4/m012 and b 3.2 10 5 m3/mol. Constants I Periodic Table Part A Determine the pressure in 1.5 mol of the gas at 1 oc if its volume is 0.41 L , calculated using the van der Waals equation. Express your answer using two significant figures. P = 74.53 Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining Part B Determine the pressure in 1.5 mol of the gas at 1 oc if its volume is 0.41 L , calculated using the ideal gas law.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


