Question: F SP Part 1: True/False Questions (10 points total, 1 point per question) TF At constant temperature, the heat of a system (AQ) is equal
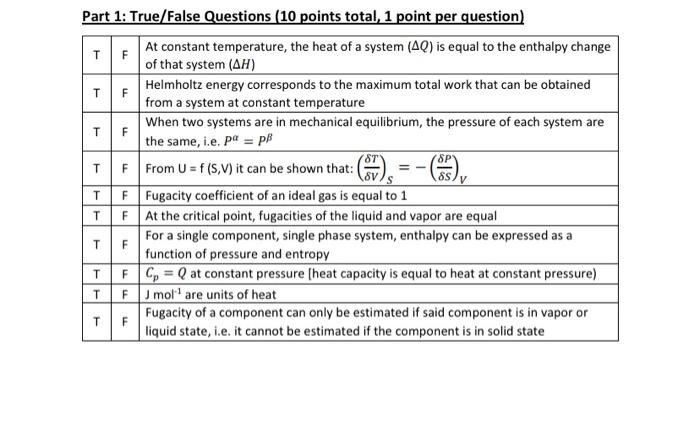
F SP Part 1: True/False Questions (10 points total, 1 point per question) TF At constant temperature, the heat of a system (AQ) is equal to the enthalpy change of that system (AH) Helmholtz energy corresponds to the maximum total work that can be obtained from a system at constant temperature When two systems are in mechanical equilibrium, the pressure of each system are TF the same, i.e. pa = pl T F From U = f (S.V) it can be shown that: SS TF Fugacity coefficient of an ideal gas is equal to 1 TF At the critical point, fugacities of the liquid and vapor are equal TF For a single component, single phase system, enthalpy can be expressed as a function of pressure and entropy T F C = Q at constant pressure [heat capacity is equal to heat at constant pressure) TF mollare units of heat TF Fugacity of a component can only be estimated if said component is in vapor or liquid state, i.e. it cannot be estimated if the component is in solid state V T
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


