Question: f7 Peptides can be separated using an ion-exchange column based on their isoelectric (pl) values. At which pH values would two different peptides, one with
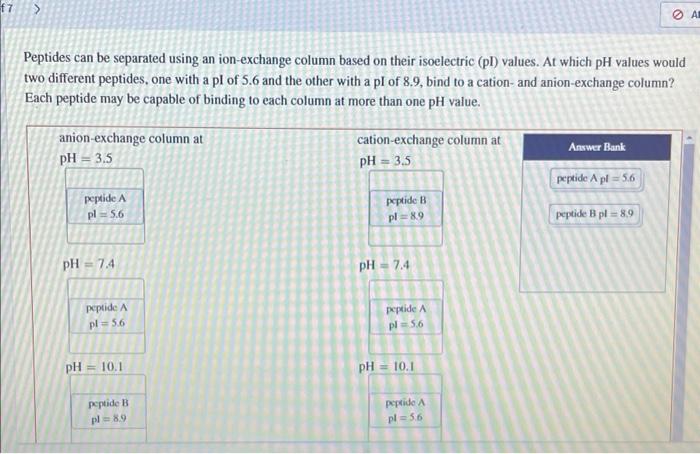
f7 Peptides can be separated using an ion-exchange column based on their isoelectric (pl) values. At which pH values would two different peptides, one with a pl of 5.6 and the other with a pl of 8.9, bind to a cation and anion-exchange column? Each peptide may be capable of binding to each column at more than one pH value. anion-exchange column at pH = 3.5 cation-exchange column at pH = 3.5 Answer Bank peptide A pl = 56 peptide A pl = 5.6 peptide B pl = 89 peptide Bpl = 89 pH = 7.4 pH = 7.4 peptide A pl= 5.6 peptide A pl = 56 pH = 10.1 pH = 10.1 peptide B pl89 peptide A pl=56
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


