Question: Fe(II) can be precipitated from a slightly basic aqueous solution by bubbling oxygen through the solution, which converts Fe(II) to insoluble Fe(Iii): 4Fe(OH)+(aq)+4OH(aq)+O2(g)+2H2O(l)4Fe(OH)3(s) How many
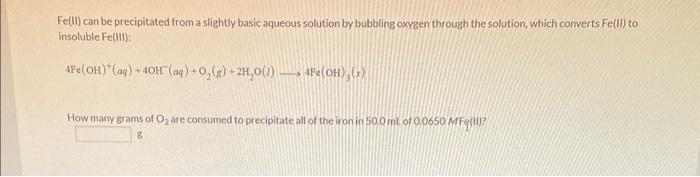
Fe(II) can be precipitated from a slightly basic aqueous solution by bubbling oxygen through the solution, which converts Fe(II) to insoluble Fe(Iii): 4Fe(OH)+(aq)+4OH(aq)+O2(g)+2H2O(l)4Fe(OH)3(s) How many grams of O2 are consumed to precipitate all of the iron in 500mL of 0.0650MF (II)? g
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


