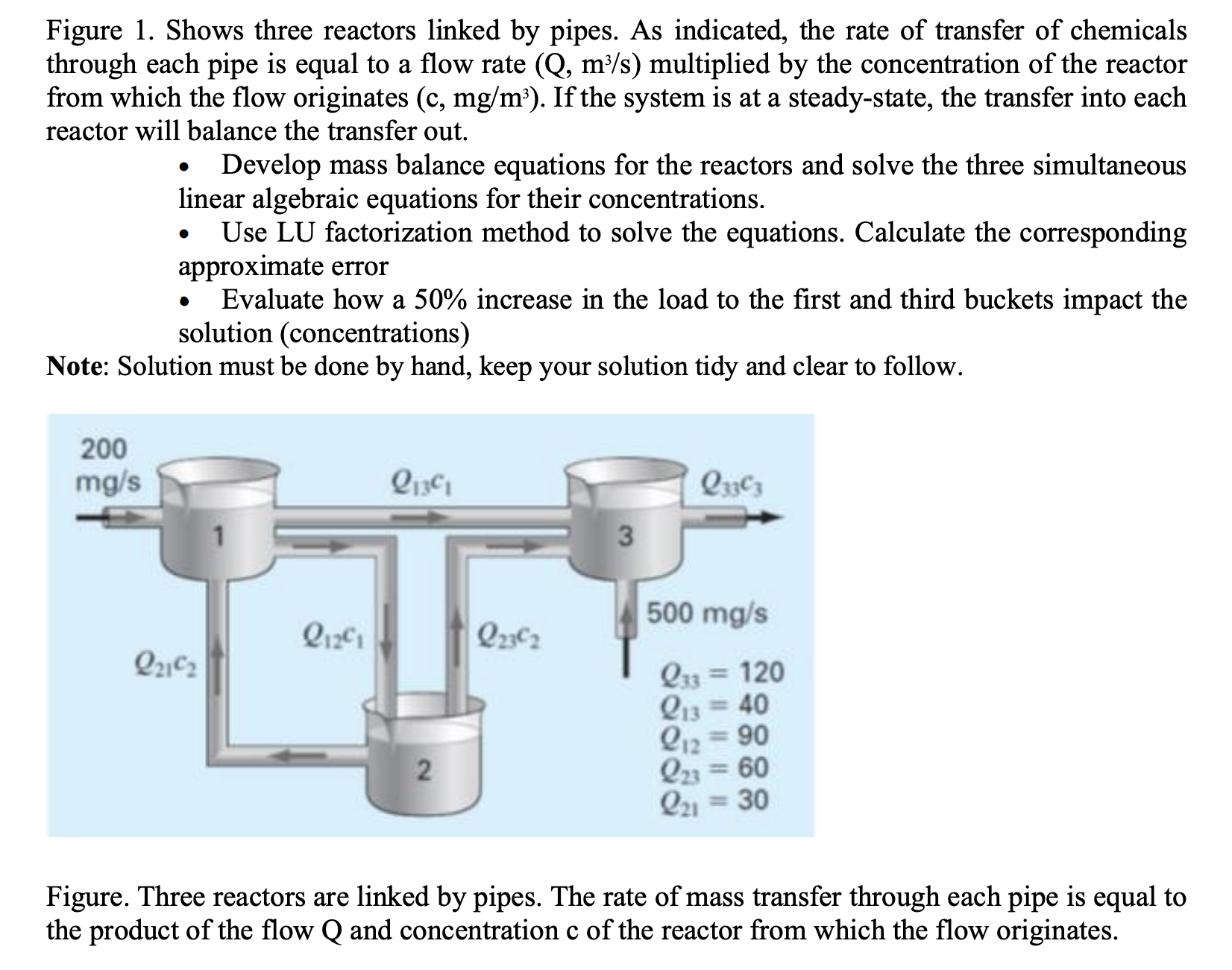
Question: Figure 1 . Shows three reactors linked by pipes. As indicated, the rate of transfer of chemicals through each pipe is equal to a flow
Figure Shows three reactors linked by pipes. As indicated, the rate of transfer of chemicals through each pipe is equal to a flow rate multiplied by the concentration of the reactor from which the flow originates If the system is at a steadystate, the transfer into each reactor will balance the transfer out.
Develop mass balance equations for the reactors and solve the three simultaneous linear algebraic equations for their concentrations.
Use LU factorization method to solve the equations. Calculate the corresponding approximate error
Evaluate how a increase in the load to the first and third buckets impact the solution concentrations
Note: Solution must be done by hand, keep your solution tidy and clear to follow.
Figure. Three reactors are linked by pipes. The rate of mass transfer through each pipe is equal to the product of the flow and concentration of the reactor from which the flow originates.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


