Question: fill the table and find final pressure The gas phase reaction below takes place in a batch constant volume reactor. After completing the table, calculate
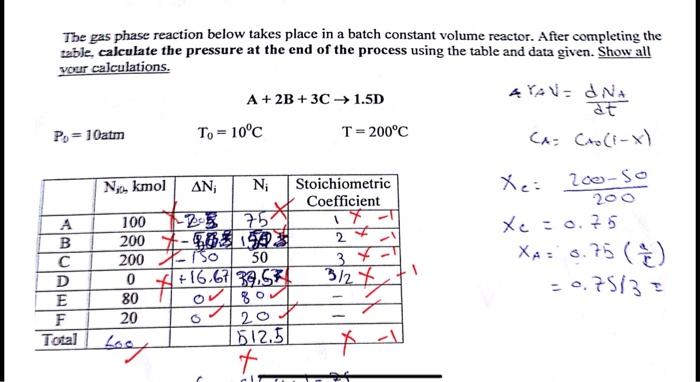
The gas phase reaction below takes place in a batch constant volume reactor. After completing the table, calculate the pressure at the end of the process using the table and data given. Show all your calculations. A + 2B+3C 1.5D #YAVE ONA at Po = 10atm T. = 10C T = 200C CA Cro(1-x) Xe: 200-SO No kmol AN Ni Stoichiometric Coefficient 100 75 17 2007-108 A3 2 2001-750 50 3 0 +16.67 39.57 3/27 80 080 A B D E F X.: A. 15 XA: 3. -0.75/33 8.75 (8) 20 207 Total Lost - Lood 612.5 t. X
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


