Question: Find the inlet temperature range to study this process Problem Statement: Production of Acetone from Isopropyl Alcohol The production of acetone (AC) can be carried
Find the inlet temperature range to study this process
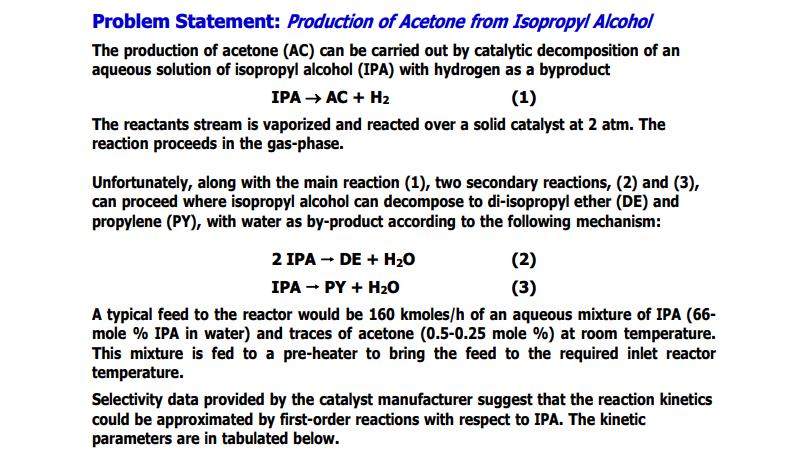
Problem Statement: Production of Acetone from Isopropyl Alcohol The production of acetone (AC) can be carried out by catalytic decomposition of an aqueous solution of isopropyl alcohol (IPA) with hydrogen as a byproduct IPA AC + H2 (1) 1 The reactants stream is vaporized and reacted over a solid catalyst at 2 atm. The reaction proceeds in the gas-phase. Unfortunately, along with the main reaction (1), two secondary reactions, (2) and (3), can proceed where isopropyl alcohol can decompose to di-isopropyl ether (DE) and propylene (PY), with water as by-product according to the following mechanism: 2 IPA - DE + H20 (2) IPA - PY + H20 (3) A typical feed to the reactor would be 160 kmoles/h of an aqueous mixture of IPA (66- mole % IPA in water) and traces of acetone (0.5-0.25 mole %) at room temperature. This mixture is fed to a pre-heater to bring the feed to the required inlet reactor temperature. Selectivity data provided by the catalyst manufacturer suggest that the reaction kinetics could be approximated by first-order reactions with respect to IPA. The kinetic parameters are in tabulated below
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


