Question: First Order Reaction. The absorbance vs. time data in Table 14-1 was recorded for a chemical reaction. The reaction was believed to follow a firstorder
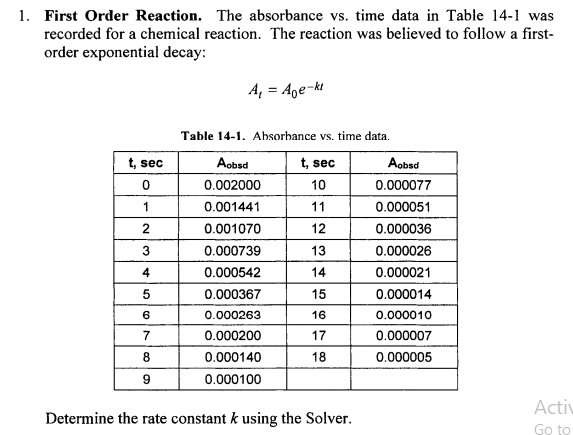
First Order Reaction. The absorbance vs. time data in Table 14-1 was recorded for a chemical reaction. The reaction was believed to follow a firstorder exponential decay: At=A0ekt Table 14-1. Absorbance vs. time data. Determine the rate constant k using the Solver
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


