Question: first photo is correct need help with the second photo. A mixture of methanol (methyl alcohol) and water contains 70.00% water by mass. Ideal Specific
first photo is correct need help with the second photo.
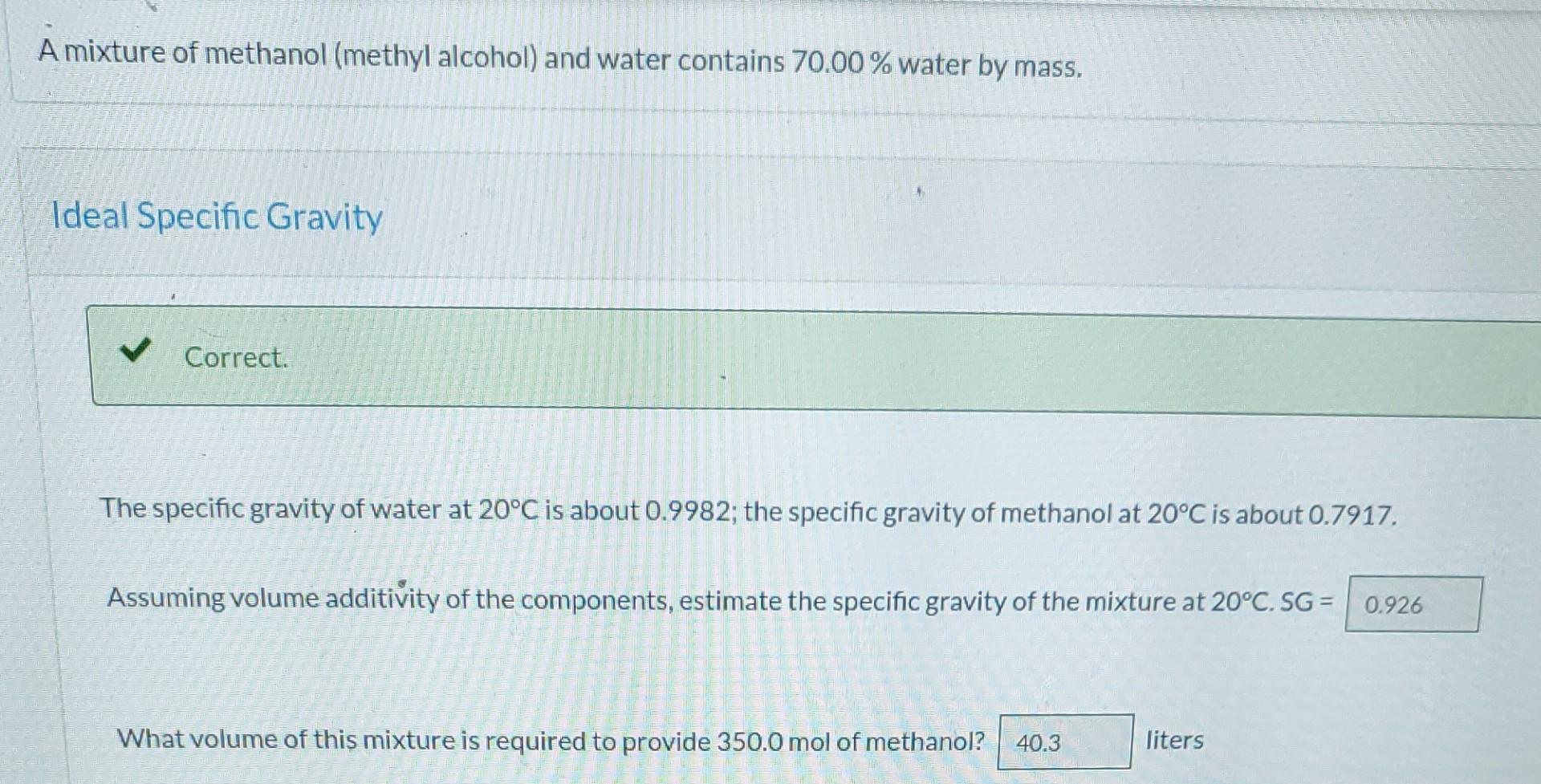
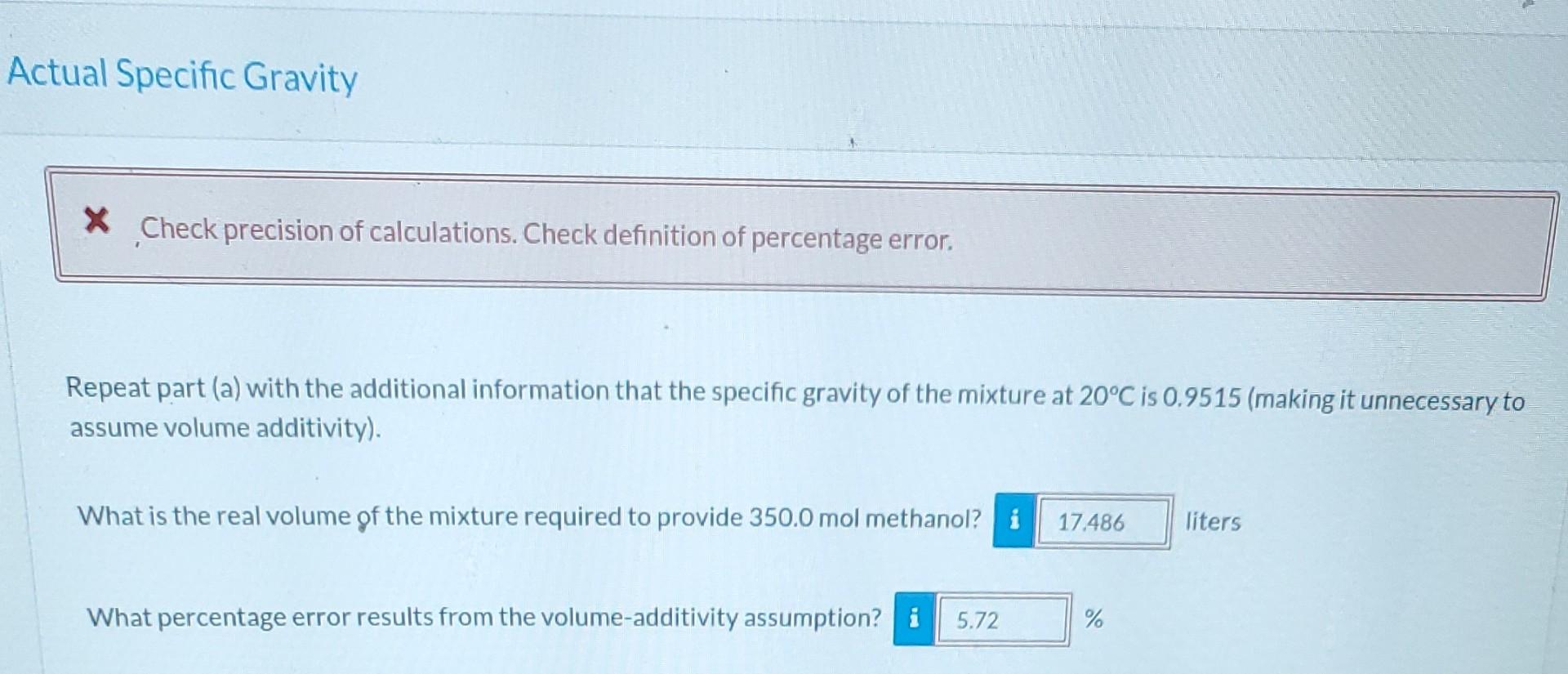
A mixture of methanol (methyl alcohol) and water contains 70.00% water by mass. Ideal Specific Gravity Correct. The specific gravity of water at 20C is about 0.9982; the specific gravity of methanol at 20C is about 0.7917. Assuming volume additivity of the components, estimate the specific gravity of the mixture at 20C. SG = 0.926 What volume of this mixture is required to provide 350.0 mol of methanol? 40.3 liters Actual Specific Gravity X Check precision of calculations. Check definition of percentage error. Repeat part (a) with the additional information that the specific gravity of the mixture at 20C is 0.9515 (making it unnecessary to assume volume additivity). What is the real volume of the mixture required to provide 350.0 mol methanol? i 17.486 liters What percentage error results from the volume-additivity assumption i 5.72 %
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


