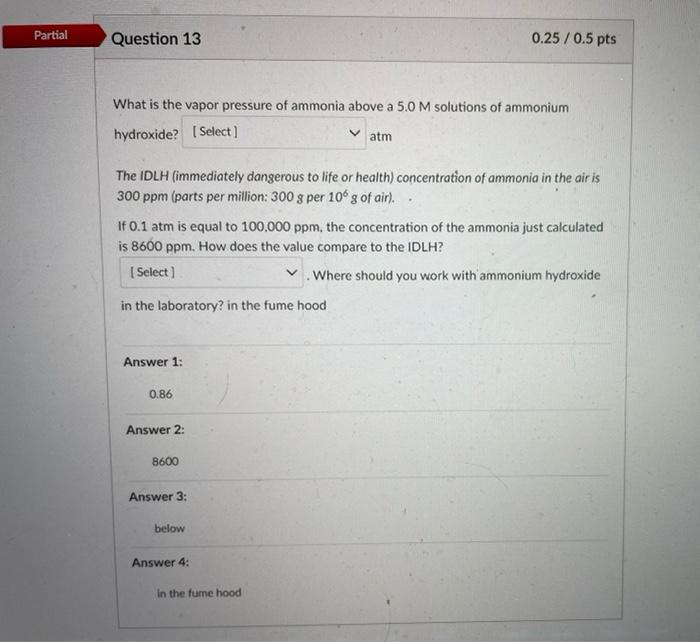
Question: First picture is my answer but something wrong so I want to get the fully correct answer. Partial Question 13 0.25 / 0.5 pts What
![solutions of ammonium hydroxide? (Select] atm The IDLH (immediately dangerous to life](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8e7b51edae_27666f8e7b4baa50.jpg)
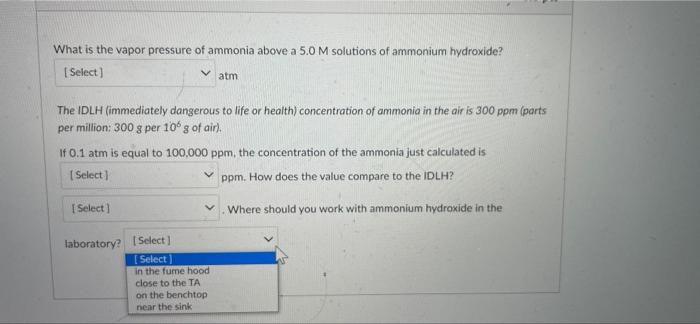
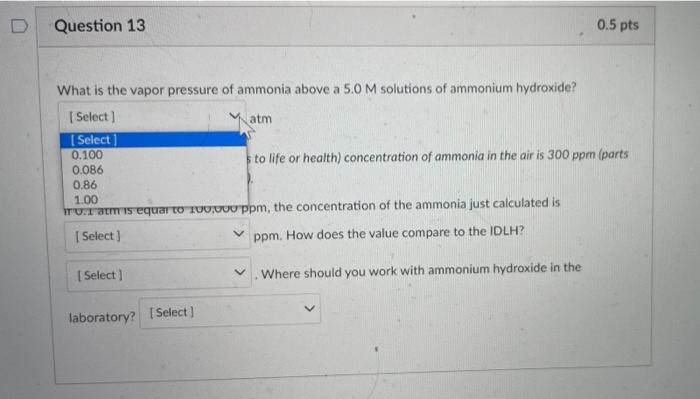
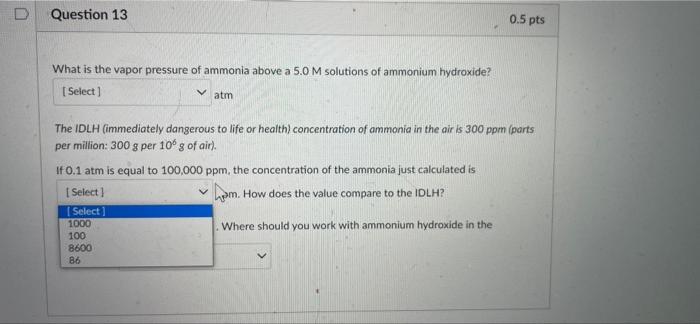
Partial Question 13 0.25 / 0.5 pts What is the vapor pressure of ammonia above a 5.0 M solutions of ammonium hydroxide? (Select] atm The IDLH (immediately dangerous to life or health) concentration of ammonia in the air is 300 ppm (parts per million: 300 g per 10 g of air). If 0.1 atm is equal to 100,000 ppm, the concentration of the ammonia just calculated is 8600 ppm. How does the value compare to the IDLH? Select) Where should you work with ammonium hydroxide in the laboratory in the fume hood Answer 1: 0.86 Answer 2: 8600 Answer 3: below Answer 4: in the fume hood D Question 13 0.5 pts What is the vapor pressure of ammonia above a 5.0 M solutions of ammonium hydroxide? Select) atm Select 0.100 sto life or health) concentration of ammonia in the air is 300 ppm (parts 0.086 0.86 1.00 ITUT aris equar TO TUUUUU ppm, the concentration of the ammonia just calculated is Select ] I ppm. How does the value compare to the IDLH? Select) Where should you work with ammonium hydroxide in the laboratory? Select] D Question 13 0.5 pts What is the vapor pressure of ammonia above a 5.0 M solutions of ammonium hydroxide? [Select ] atm The IDLH (immediately dangerous to life or health) concentration of ammonia in the air is 300 ppm (parts per million: 300 g per 100 g of air). If 0.1 atm is equal to 100,000 ppm, the concentration of the ammonia just calculated is Select ! ham. How does the value compare to the IDLH? Select 1000 Where should you work with ammonium hydroxide in the 100 8600 86 What is the vapor pressure of ammonia above a 5.0M solutions of ammonium hydroxide? Select) atm The IDLH (immediately dangerous to life or health) concentration of ammonia in the air is 300 ppm (parts per million: 300 g per 10g of air). If 0.1 atm is equal to 100,000 ppm, the concentration of the ammonia just calculated is [Select) ppm. How does the value compare to the IDLH? Where should you work with ammonium hydroxide in the Select] Select below above the same What is the vapor pressure of ammonia above a 5.0 M solutions of ammonium hydroxide? [Select) atm The IDLH (immediately dangerous to life or health) concentration of ammonia in the air is 300 ppm (parts per million: 300 g per 10 g of air) If 0.1 atm is equal to 100,000 ppm, the concentration of the ammonia just calculated is Select) ppm. How does the value compare to the IDLH? Select) Where should you work with ammonium hydroxide in the laboratory? Select [ Select in the fume hood close to the TA on the benchtop near the sink
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


