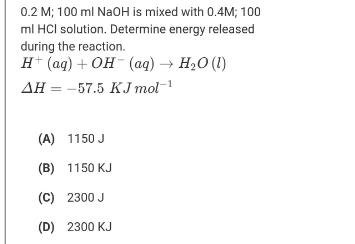Question: 0.2 M; 100 ml NAOH is mixed with 0.4M; 100 ml HCI solution. Determine energy released during the reaction. H (aq) + OH (aq)
0.2 M; 100 ml NAOH is mixed with 0.4M; 100 ml HCI solution. Determine energy released during the reaction. H (aq) + OH (aq) H,0 (1) AH = -57.5 KJ mol-1 (A) 1150 J (B) 1150 KJ (C) 2300 J (D) 2300 KJ
Step by Step Solution
3.58 Rating (159 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts