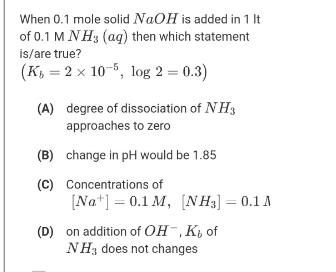
Question: When 0.1 mole solid NaOH is added in 1 It of 0.1 M NH3 (aq) then which statement is/are true? (K, = 2 x
When 0.1 mole solid NaOH is added in 1 It of 0.1 M NH3 (aq) then which statement is/are true? (K, = 2 x 10-5, log 2 = 0.3) (A) degree of dissociation of NH3 approaches to zero (B) change in pH would be 1.85 (C) Concentrations of [Na*] = 0.1 M, [NH3] = 0.1 A (D) on addition of OH, K, of NH3 does not changes
Step by Step Solution
3.46 Rating (156 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts