Question: Five thermodynamic processes are illustrated below. All five processes are for the same ideal gas startif and the same initial pressure and temperature ( P,
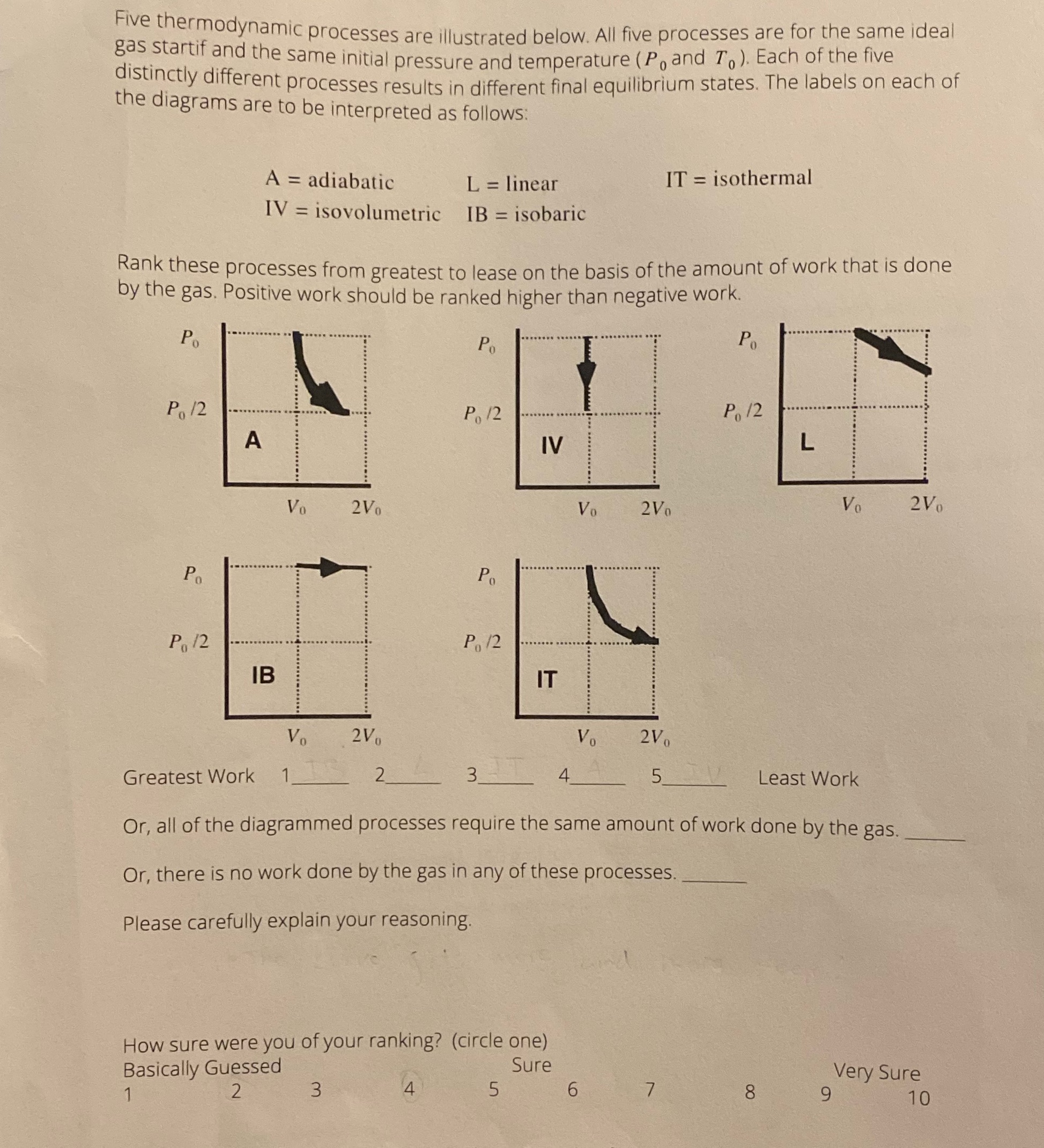
Five thermodynamic processes are illustrated below. All five processes are for the same ideal gas startif and the same initial pressure and temperature ( P, and To ). Each of the five distinctly different processes results in different final equilibrium states. The labels on each of the diagrams are to be interpreted as follows: A = adiabatic L = linear IT = isothermal IV = isovolumetric IB = isobaric Rank these processes from greatest to lease on the basis of the amount of work that is done by the gas. Positive work should be ranked higher than negative work. P. . . ..... Po Po .. .. ...... Po 12 Po 12 Po 12 A IV L .. ......... Vo 2Vo Vo 2Vo Vo 2Vo Po Po . .......-........ ..com Po 12 Po 12 ... IB IT Vo 2Vo Vo 2Vo Greatest Work 1 3 5 Least Work Or, all of the diagrammed processes require the same amount of work done by the gas. Or, there is no work done by the gas in any of these processes. Please carefully explain your reasoning. How sure were you of your ranking? (circle one) Basically Guessed Sure Very Sure 2 3 4 5 6 7 8 9 10
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


