Question: for #2 use the dimensional analysis DATA: Determining drop volume 1 2 Trial Iml 7 ML Initial volume (mL) O ML Final volume (mL) ImL

for #2 use the dimensional analysis
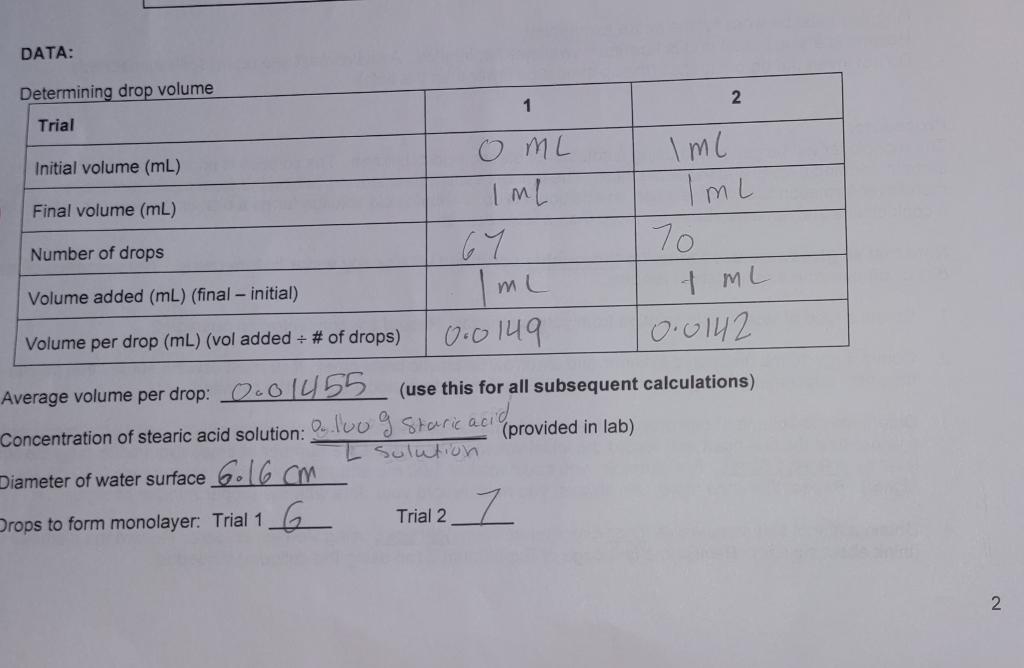
DATA: Determining drop volume 1 2 Trial Iml 7 ML Initial volume (mL) O ML Final volume (mL) ImL Iml Number of drops 67 70 Volume added (mL) (final - initial) Imu Volume per drop (mL) (vol added + # of drops) 0.0149 10.0142 Average volume per drop: 0.01455 (use this for all subsequent calculations) Concentration of stearic acid solution: 0. loug Staric acid (provided in lab) I solution Diameter of water surface 6.16 cm Drops to form monolayer: Trial 1 G Trial 2 I 2 1. Calculate the surface area of the monolayer (= water surface) (in cm2) using the calipers measurement. A = ter 2. Calculate the grams of stearic acid molecules used to form the monolayer in each trial via dimensional analysis: start with # of drops to form monolayer x avg. volume per drop (mL/drop). You will be using the calculation you entered below the table on p. 2, and you will need to use the concentration of the stearic acid solution. Do this for each trial Trial 1: Trial 2: Average
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


