Question: For a first order irreversible solid gas non-catalytic reaction A (g) + bB (s) dD (g) + pP (s) the reagent particles are long
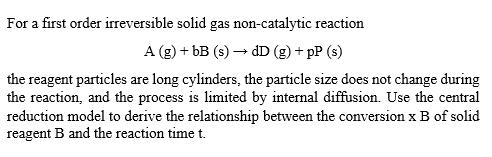
For a first order irreversible solid gas non-catalytic reaction A (g) + bB (s) dD (g) + pP (s) the reagent particles are long cylinders, the particle size does not change during the reaction, and the process is limited by internal diffusion. Use the central reduction model to derive the relationship between the conversion x B of solid reagent B and the reaction time t.
Step by Step Solution
3.52 Rating (159 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


