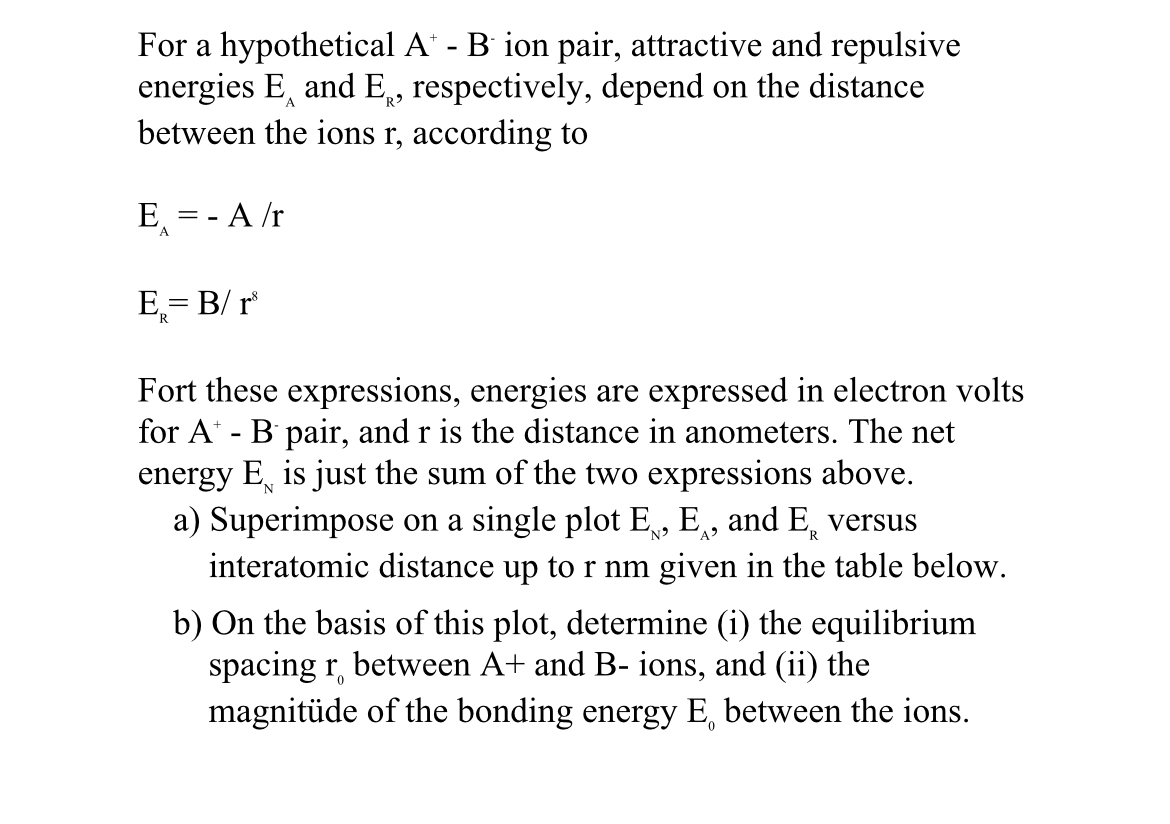
Question: For a hypothetical A + - B ion pair, attractive and repulsive energies E A and E R , respectively, depend on the distance between
For a hypothetical ion pair, attractive and repulsive energies and respectively, depend on the distance between the ions r according to
Fort these expressions, energies are expressed in electron volts for pair, and r is the distance in anometers. The net energy is just the sum of the two expressions above.
a Superimpose on a single plot and versus interatomic distance up to r nm given in the table below.
b On the basis of this plot, determine i the equilibrium spacing between and B ions, and ii the magnitde of the bonding energy between the ions.
A
BE
rnm
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


