Question: For a particular process, it is desired to have a gas phase stream of oxygen (O) plus equal molar flowrates of methane and water
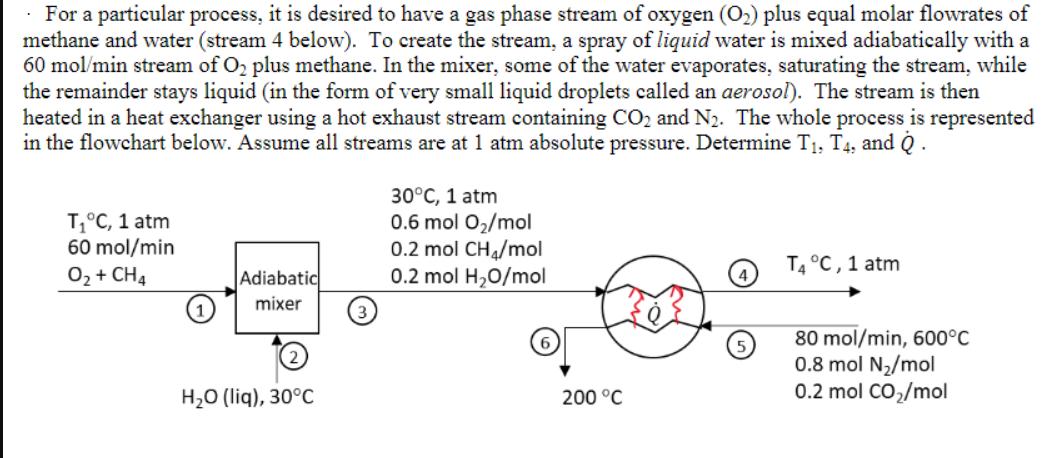
For a particular process, it is desired to have a gas phase stream of oxygen (O) plus equal molar flowrates of methane and water (stream 4 below). To create the stream, a spray of liquid water is mixed adiabatically with a 60 mol/min stream of O plus methane. In the mixer, some of the water evaporates, saturating the stream, while the remainder stays liquid (in the form of very small liquid droplets called an aerosol). The stream is then heated in a heat exchanger using a hot exhaust stream containing CO and N. The whole process is represented in the flowchart below. Assume all streams are at 1 atm absolute pressure. Determine T, T4, and Q. T C, 1 atm 60 mol/min O + CH4 Adiabatic mixer HO (liq), 30C 3 30C, 1 atm 0.6 mol O/mol 0.2 mol CH/mol 0.2 mol H0/mol 200 C T4 C, 1 atm 80 mol/min, 600C 0.8 mol N/mol 0.2 mol CO/mol
Step by Step Solution
3.47 Rating (154 Votes )
There are 3 Steps involved in it
To solve this problem we need to use the principles of thermodynamics and mass conservation First lets identify the streams and their properties Stream 1 O2 CH4 60 molmin 1 atm T1 30C Stream 2 Water 0... View full answer

Get step-by-step solutions from verified subject matter experts


