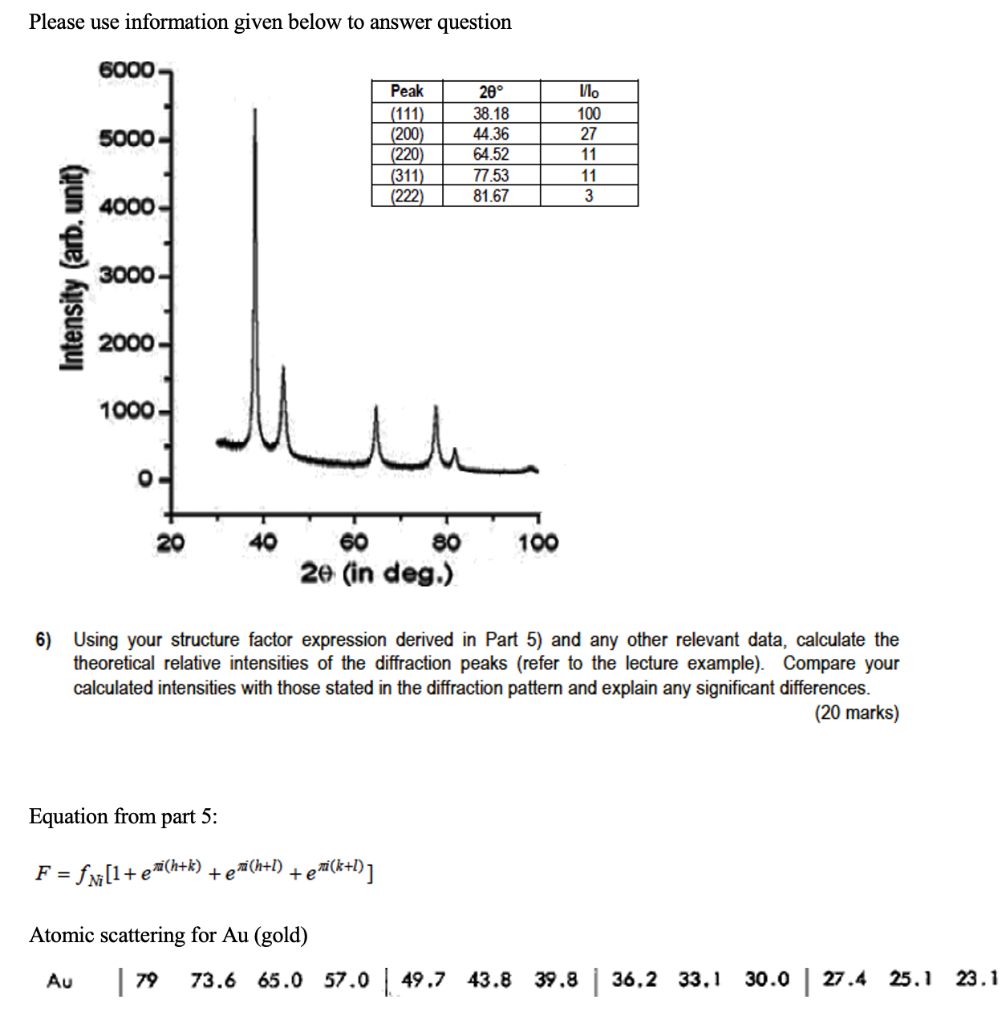
Question: For atomic scattering factor, the associated values are: Please use information given below to answer question 6000 20 38.18 5000 Peak (111) (200) (220) (311)
For atomic scattering factor, the associated values are: 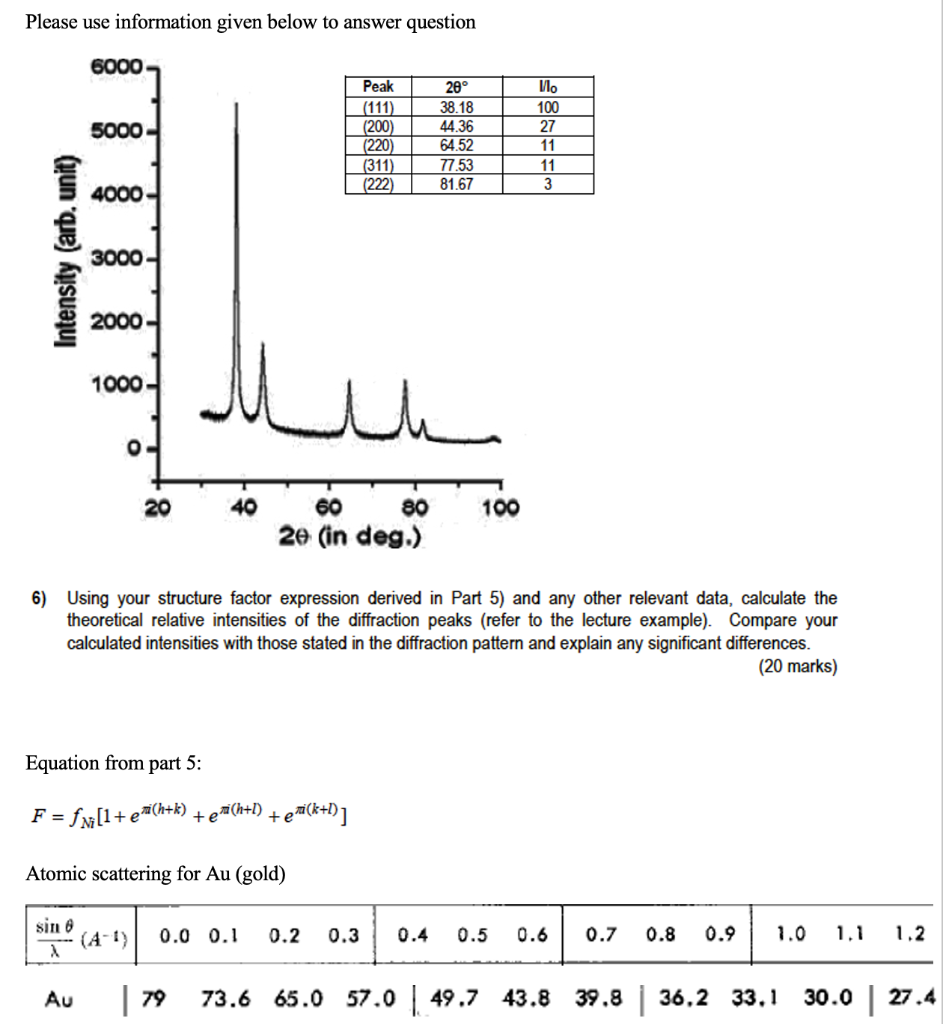
Please use information given below to answer question 6000 20 38.18 5000 Peak (111) (200) (220) (311) (222) 44.36 64.52 77.53 81.67 100 27 11 11 3 4000 Intensity (arb, unit) 3000- 2000 1000 0 20 40 100 60 80 2e (in deg.) 6) Using your structure factor expression derived in Part 5) and any other relevant data, calculate the theoretical relative intensities of the diffraction peaks (refer to the lecture example). Compare your calculated intensities with those stated in the diffraction pattern and explain any significant differences. (20 marks) Equation from part 5: F = f Ni[l+exi(h+k) teni(h+l) + eni(k+l)] Atomic scattering for Au (gold) 73.6 65.0 57.0 | 49.7 43.8 Au | 79 39.8 | 36,2 33.1 30.0 27.4 25.1 23.1 Please use information given below to answer question 6000 5000 LLL Peak (111) (200) (220) (311) 20 38.18 44.36 64.52 77.53 81.67 100 27 11 11 3 4000 (222) Intensity (arb, unit) 3000- 2000- 1000- 0 - 20 40 100 60 80 2e (in deg.) 6) Using your structure factor expression derived in Part 5) and any other relevant data, calculate the theoretical relative intensities of the diffraction peaks (refer to the lecture example). Compare your calculated intensities with those stated in the diffraction pattern and explain any significant differences. (20 marks) Equation from part 5: F = fm [1+ [+e(h+k) +e (h+1) +ea(k+l)] Atomic scattering for Au (gold) sin 6 (4-1) X 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 1.1 1.2 Au | 79 73.6 65.0 57.0 | 49.7 43.8 39.8 | 36,2 33.1 30.0 | 27.4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


