Question: For b), what's the difference between wavefunction and wavefunction squared? How do I identify the radial and angular nodes based on the graphs? (b) Give
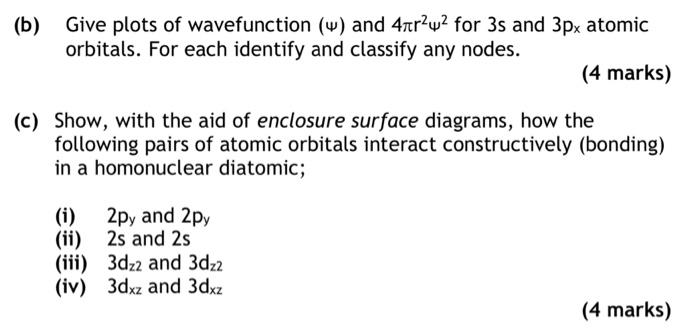
(b) Give plots of wavefunction ( ) and 4r22 for 3s and 3px atomic orbitals. For each identify and classify any nodes. (4 marks) (c) Show, with the aid of enclosure surface diagrams, how the following pairs of atomic orbitals interact constructively (bonding) in a homonuclear diatomic; (i) 2py and 2py (ii) 2s and 2s (iii) 3dz2 and 3dz2 (iv) 3dxz and 3dxz (b) Give plots of wavefunction ( ) and 4r22 for 3s and 3px atomic orbitals. For each identify and classify any nodes. (4 marks) (c) Show, with the aid of enclosure surface diagrams, how the following pairs of atomic orbitals interact constructively (bonding) in a homonuclear diatomic; (i) 2py and 2py (ii) 2s and 2s (iii) 3dz2 and 3dz2 (iv) 3dxz and 3dxz
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


