Question: For cases where [A] exactly doubles: - if the rate does not change, the order for A is 0 . - if the rate doubles,
![For cases where [A] exactly doubles: - if the rate does](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8f59064202_82366f8f58fd8dce.jpg)
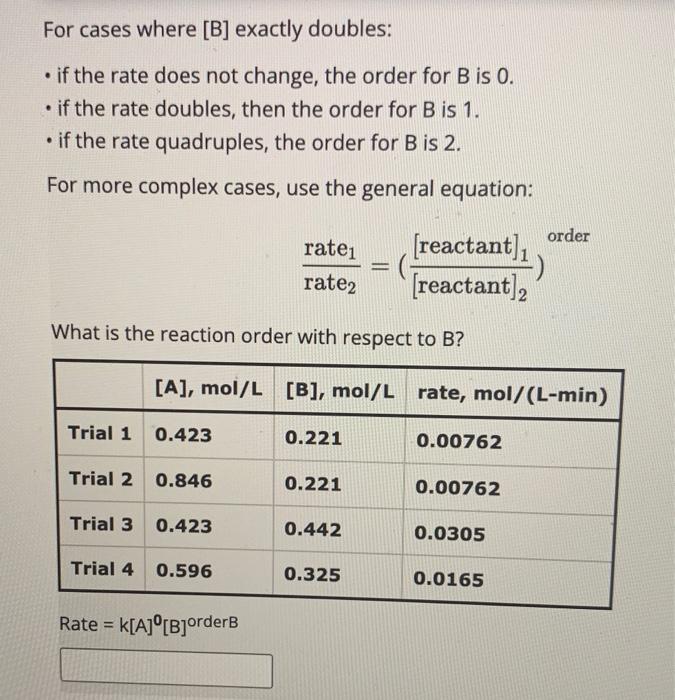
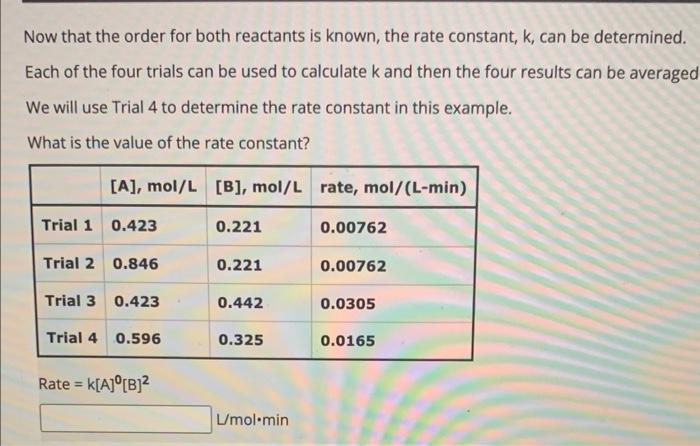
For cases where [A] exactly doubles: - if the rate does not change, the order for A is 0 . - if the rate doubles, then the order for A is 1. - if the rate quadruples, the order for A is 2. For more complex cases, use the general equation: rate2rate1=([reactant]2[reactant]1)order What is the reaction order with respect to A ? Rate =k[A]orderA[B]orderB For cases where [B] exactly doubles: - if the rate does not change, the order for B is 0 . - if the rate doubles, then the order for B is 1. - if the rate quadruples, the order for B is 2. For more complex cases, use the general equation: rate2rate1=([reactant]2[reactant]1)order What is the reaction order with respect to B ? Rate =k[A]0[B]orderB Now that the order for both reactants is known, the rate constant, k, can be determined. Each of the four trials can be used to calculate k and then the four results can be averaged We will use Trial 4 to determine the rate constant in this example. What is the value of the rate constant? Rate =k[A]0[B]2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


