Question: for classification : ionic ,molecular or aqueous solution. as well as chemical formulas begin{tabular}{|c|c|c|c|} hline Name & IncorrectChemicalFormula & CorrectChemicalFormula & Explanation hline calcium
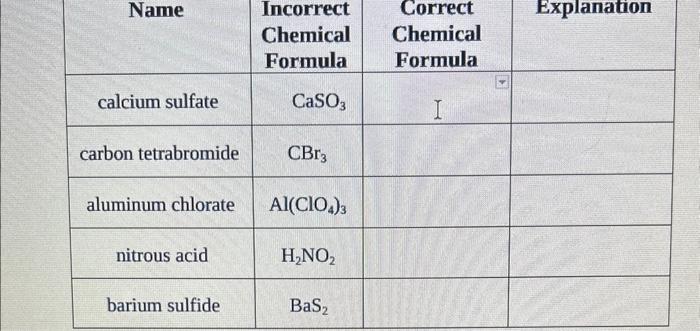
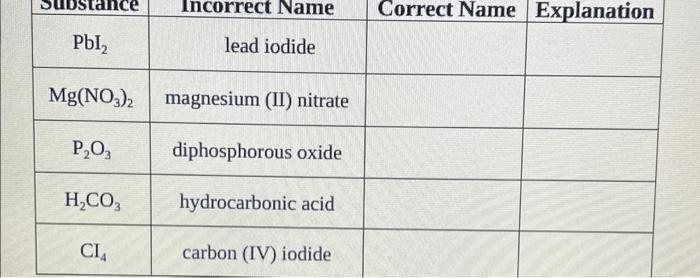
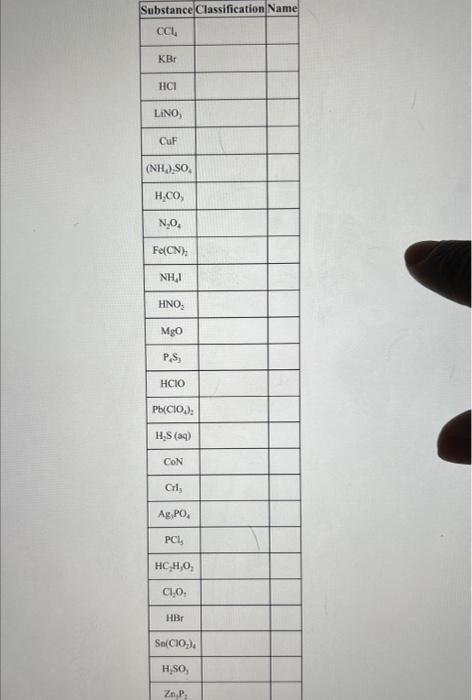
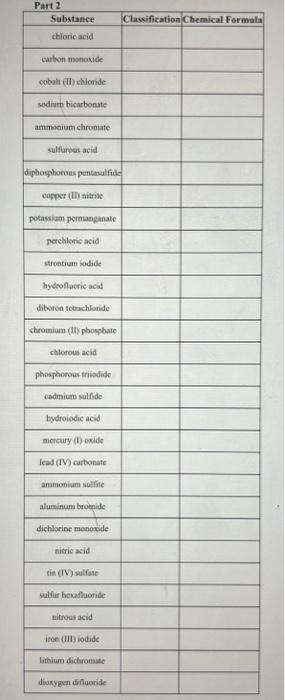
\begin{tabular}{|c|c|c|c|} \hline Name & IncorrectChemicalFormula & CorrectChemicalFormula & Explanation \\ \hline calcium sulfate & CaSO3 & I & \\ \hline carbon tetrabromide & CBr3 & & \\ \hline aluminum chlorate & Al(ClO4)3 & & \\ \hline nitrous acid & H2NO2 & & \\ \hline barium sulfide & BaS2 & & \\ \hline \end{tabular} \begin{tabular}{|c|c|c|c|} \hline PbI2 & lead iodide & & Explanation \\ \hline Mg(NO3)2 & magnesium (II) nitrate & & \\ \hline P2O3 & diphosphorous oxide & & \\ \hline H2CO3 & hydrocarbonic acid & & \\ \hline CI4 & carbon (IV) iodide & & \\ \hline \end{tabular} Part 2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


