Question: For each chemical reaction scheme below, write down a system of ordinary differential equations describing the dynamics assuming mass action kinetics. Please use cX to
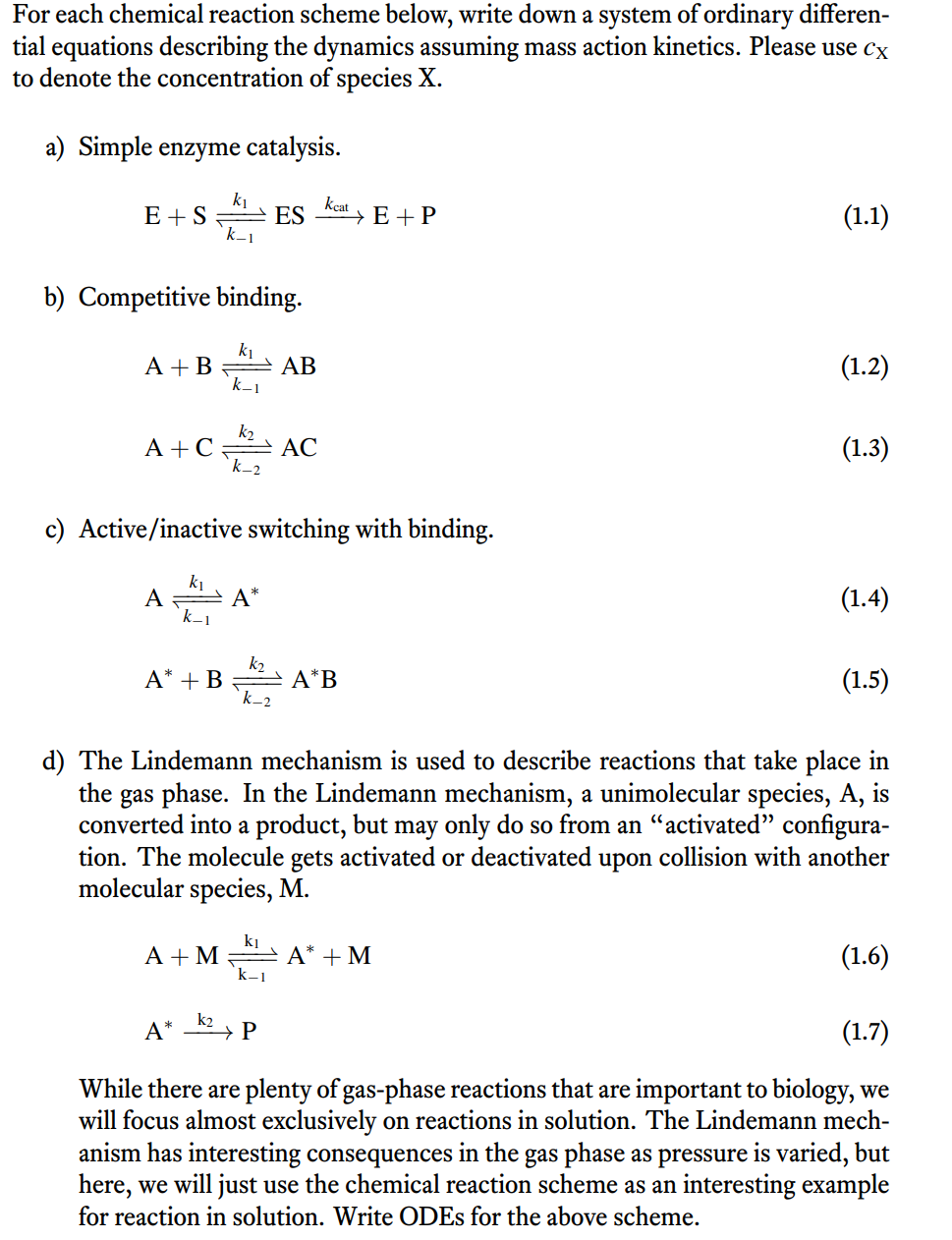

For each chemical reaction scheme below, write down a system of ordinary differential equations describing the dynamics assuming mass action kinetics. Please use cX to denote the concentration of species X. a) Simple enzyme catalysis. b) Competitive binding. A+Ck2N1k2AC c) Active/inactive switching with binding. Ak1k1AA+Bk2k2AB d) The Lindemann mechanism is used to describe reactions that take place in the gas phase. In the Lindemann mechanism, a unimolecular species, A, is converted into a product, but may only do so from an "activated" configuration. The molecule gets activated or deactivated upon collision with another molecular species, M. A+Mk1k1A+MAk2P While there are plenty of gas-phase reactions that are important to biology, we will focus almost exclusively on reactions in solution. The Lindemann mechanism has interesting consequences in the gas phase as pressure is varied, but here, we will just use the chemical reaction scheme as an interesting example for reaction in solution. Write ODEs for the above scheme. e) Multiple routes to the same place. AB+Ck3k3ABC We will show that there are restrictions on the values that the rate constants may take later in the term
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


