Question: For each compound below: -draw the Lewis structure - calculate the molecular weight -identify the intermolecular forces that exist between molecules in a pure sample.
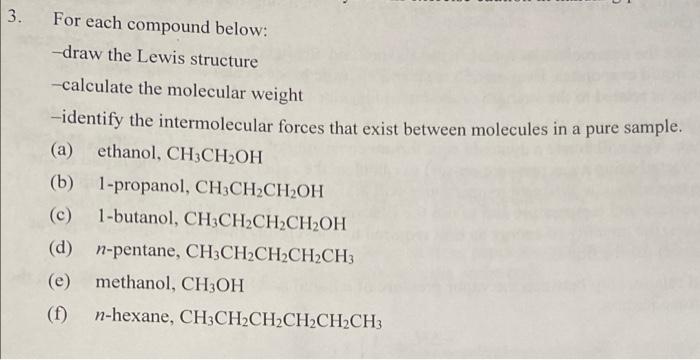
For each compound below: -draw the Lewis structure - calculate the molecular weight -identify the intermolecular forces that exist between molecules in a pure sample. (a) ethanol, CH3CH2OH (b) 1-propanol, CH3CH2CH2OH (c) 1-butanol, CH3CH2CH2CH2OH (d) n-pentane, CH3CH2CH2CH2CH3 (e) methanol, CH3OH (f) n-hexane, CH3CH2CH2CH2CH2CH3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


