Question: REPORT SUMMARY Complete Report Table EIMF. 1 with your calculations for the change in temperature. Table view List view 1. Draw a Lewis structure for
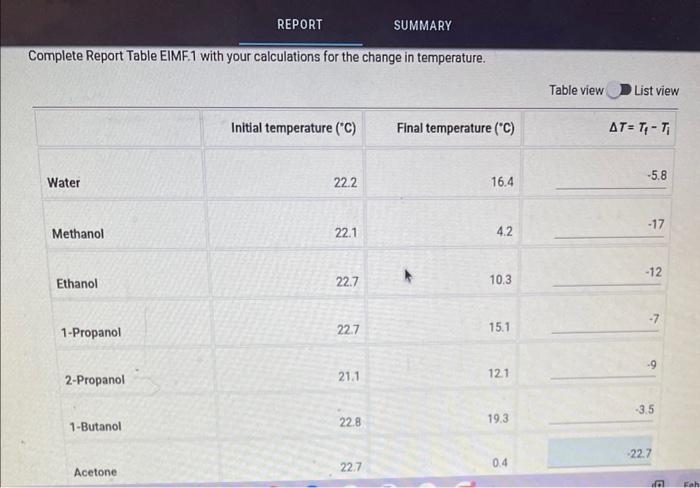

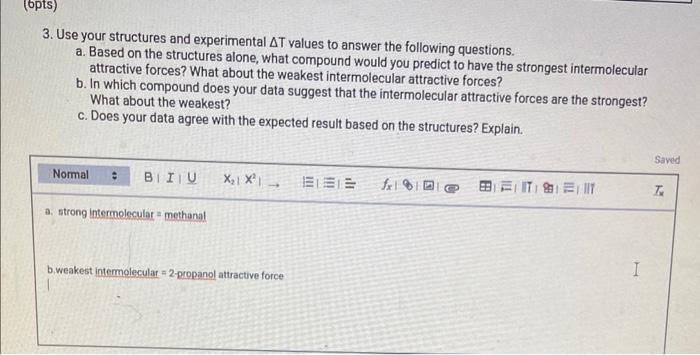
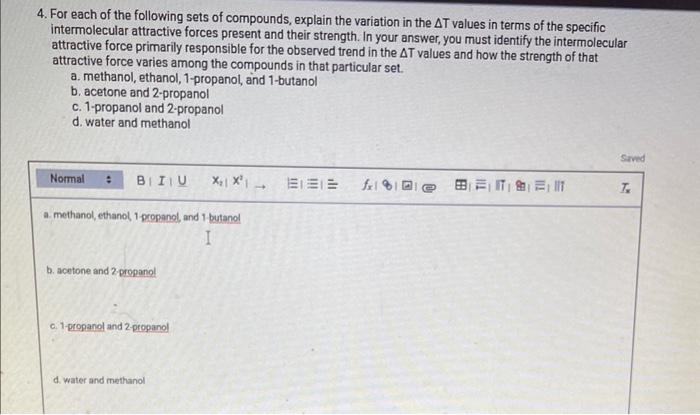
REPORT SUMMARY Complete Report Table EIMF. 1 with your calculations for the change in temperature. Table view List view 1. Draw a Lewis structure for each of the molecules used in this experiment. (Be sure to show nonbonding electrons.) You can insert images in this response by clicking on the mountain landscape icon. (3pts) 2. Briefly describe what occurs when a molecular liquid evaporates. Are any bonds within a molecule broken? Justify your reasoning. 3. Use your structures and experimental T values to answer the following questions. a. Based on the structures alone, what compound would you predict to have the strongest intermolecular attractive forces? What about the weakest intermolecular attractive forces? b. In which compound does your data suggest that the intermolecular attractive forces are the strongest? What about the weakest? c. Does your data agree with the expected result based on the structures? Explain. 4. For each of the following sets of compounds, explain the variation in the T values in terms of the specific intermolecular attractive forces present and their strength. In your answer, you must identify the intermolecular attractive force primarily responsible for the observed trend in the T values and how the strength of that attractive force varies among the compounds in that particular set. a. methanol, ethanol, 1-propanol, and 1-butanol b. acetone and 2-propanol c. 1-propanol and 2-propanol d. water and methanol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


