Question: For each initial mixture (1),(2), and (3) shown below, determine the direction the reaction will proceed to reach equilibrium Given the reaction: A2+B=A+ABKc=5.0 For each
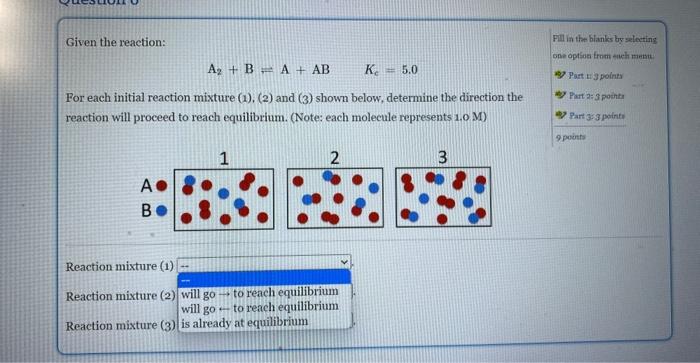
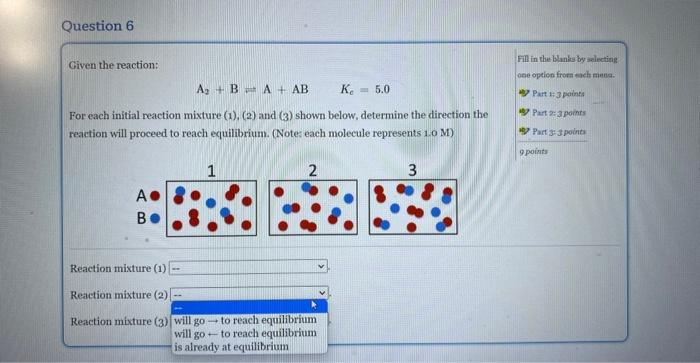
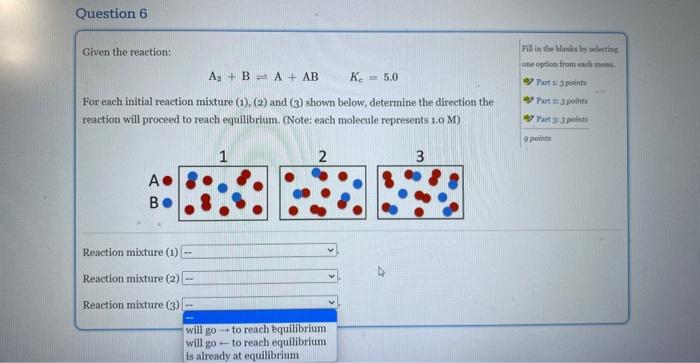
Given the reaction: A2+B=A+ABKc=5.0 For each initial reaction mixture (1), (2) and (3) shown below, determine the direction the Fill ia the blanks by soloting reaction will proceed to reach equilibrium. (Note: each molecule represents 1.0 M) A B Reaction mixture (1) Reaction mixture (2) Reaction mixture (3) Given the reaction: Fill in the blanks by selecting A2+BA+ABKc=5.0 cee opelion from each mana. For each initial reaction mixture (1), (2) and ( 3 ) shown below, determine the direction the reaction will proceed to reach equilibrium. (Notes each molecule represents 1.0M ) A B Reaction mixture (1) Reaction mixture (2) Reaction mixture (3) Given the reaction: Pill in the Hank by uelerting A2+BA+ABKc=5,0 one option from each mann. 45) Part 14 apolint For each initial reaction mixture (1),(2) and (3) shown below, determine the direction the reaction will proceed to reach equilibrium. (Note: each molecule represents 1.0 M) B 4. Partaijpointe 4 Part 353 puints 9 ponta Reaction mixture (1) Reaction mixture (2) Reaction mixture (3)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


