Question: Notice that for the dissolution of any solid, the expression for Kc will always contain the concentration of the solid in the denominator. Because the
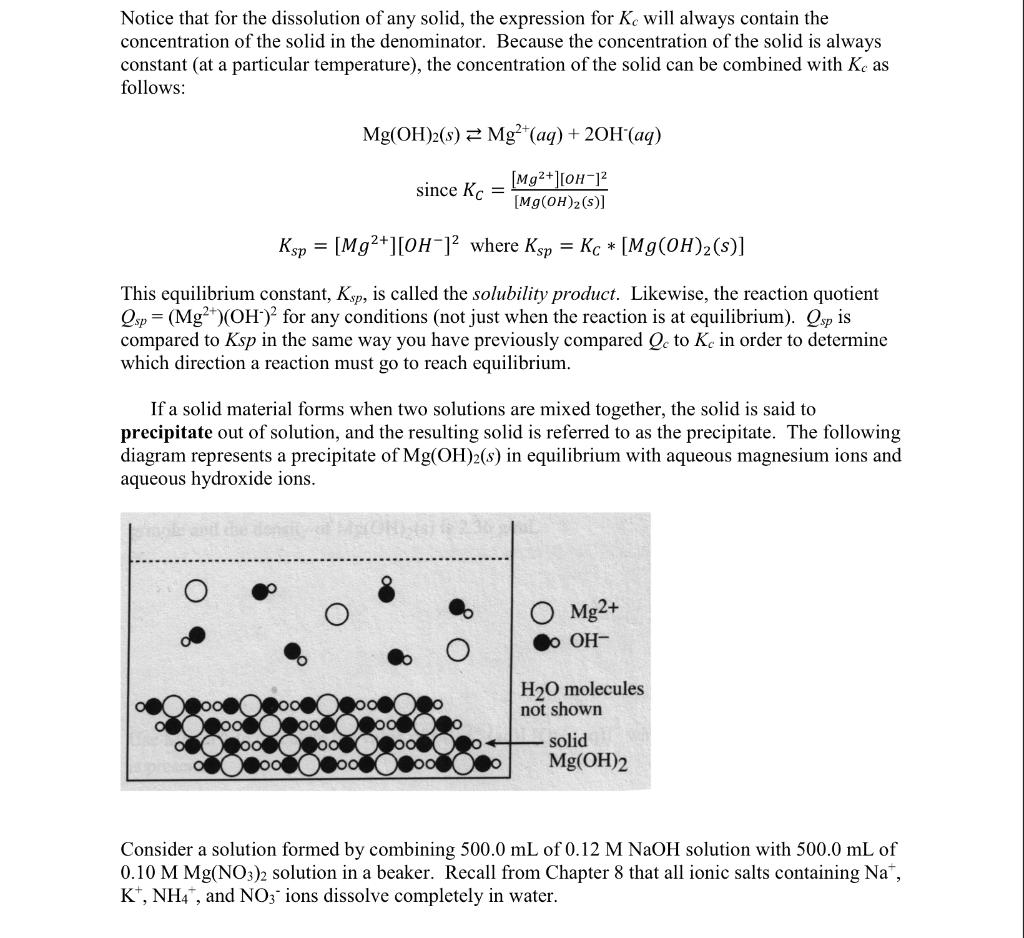

Notice that for the dissolution of any solid, the expression for Kc will always contain the concentration of the solid in the denominator. Because the concentration of the solid is always constant (at a particular temperature), the concentration of the solid can be combined with Kc as follows: Mg(OH)2(s)Mg2+(aq)+2OH(aq)sinceKC=[Mg(OH)2(s)][Mg2+][OH]2Ksp=[Mg2+][OH]2whereKsp=KC[Mg(OH)2(s)] This equilibrium constant, Ksp, is called the solubility product. Likewise, the reaction quotient Qsp=(Mg2+)(OH)2 for any conditions (not just when the reaction is at equilibrium). Qsp is compared to Ksp in the same way you have previously compared Qc to Kc in order to determine which direction a reaction must go to reach equilibrium. If a solid material forms when two solutions are mixed together, the solid is said to precipitate out of solution, and the resulting solid is referred to as the precipitate. The following diagram represents a precipitate of Mg(OH)2(s) in equilibrium with aqueous magnesium ions and aqueous hydroxide ions. Consider a solution formed by combining 500.0mL of 0.12MNaOH solution with 500.0mL of 0.10MMg(NO3)2 solution in a beaker. Recall from Chapter 8 that all ionic salts containing Na+, K+,NH4+, and NO3ions dissolve completely in water. 1. Show that the initial concentration of Mg2+ (i.e., right after you combine the Mg(NO3)2 and NaOH) is 0.05M. 2. What is the initial concentration of OH? 3. Write the expression for the reaction quotient, Qsp, for this reaction: Mg(OH)2(s)Mg2+(aq)+2OH(aq) Calculate the value of Qsp for this mixture
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


