Question: For each part below, use the data below to calculate G at 225K for each of the following reactions. Then use the data in the
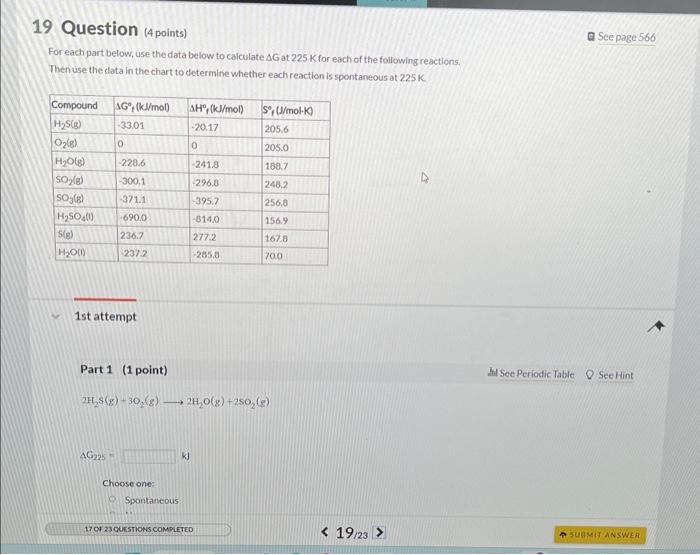
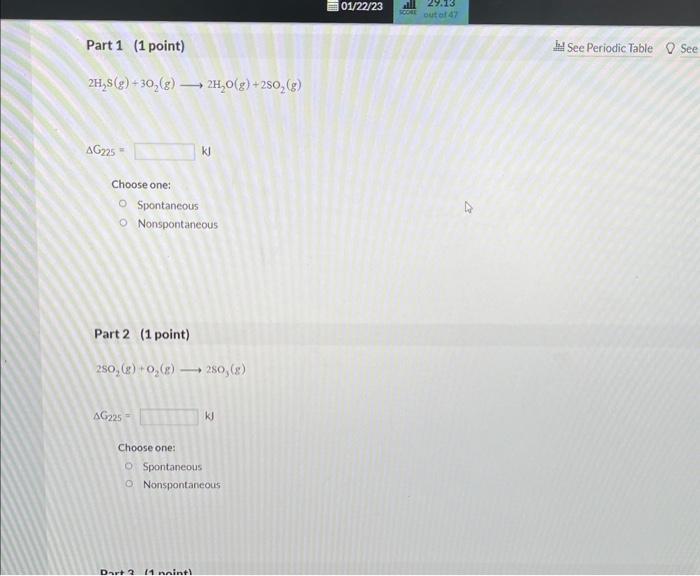

For each part below, use the data below to calculate G at 225K for each of the following reactions. Then use the data in the chart to determine whether each reaction is spontaneous at 225K. 1st attempt Part 1 (1 point) 2H2S(g)+3O2(g)2H2O(g)+2SO2(g) AC225 kJ Choose one: Spontancous: 2H2S(g)+3O2(g)2H2O(g)+2SO2(g) G225=kJ Choose one: Spontaneous Nonspontaneous Part 2 (1 point) 2SO2(g)+O2(g)2SO3(g) SO3(g)+H2O(l)H2SO4(l)G225=kJ Choose one: Spontaneous Nonspontaneous Part 4 (1 point) S(g)+O2(g)SO2(g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


