Question: Suppose you performed a similar experiment using diphenyl ether as your solvent. Given the data in the table, what would be the freezing point
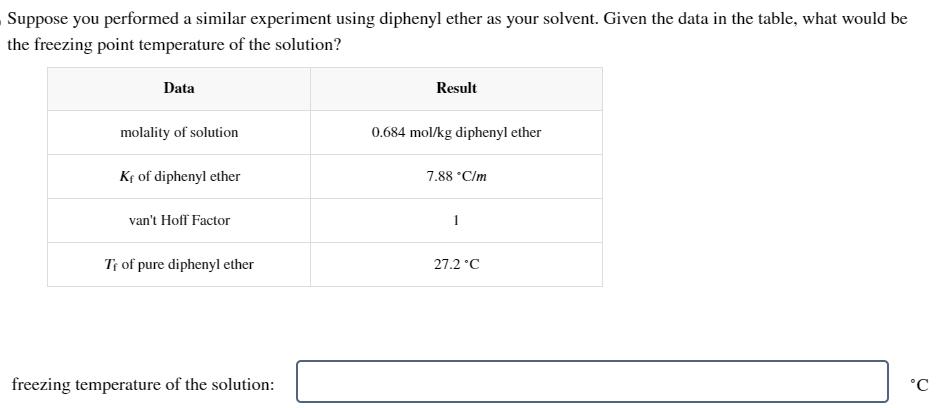
Suppose you performed a similar experiment using diphenyl ether as your solvent. Given the data in the table, what would be the freezing point temperature of the solution? Data Result molality of solution 0.684 mol/kg diphenyl ether Kf of diphenyl ether van't Hoff Factor T of pure diphenyl ether freezing temperature of the solution: 7.88 C/m 27.2C C
Step by Step Solution
3.41 Rating (151 Votes )
There are 3 Steps involved in it
To determine the freezing point temperature of the solution we can use the equation T Kf m i Wh... View full answer

Get step-by-step solutions from verified subject matter experts


