Question: For part ii, please show the mathematical steps. I am confused (i) What is the critical value of required for high molar mass polymers to
For part ii, please show the mathematical steps. I am confused
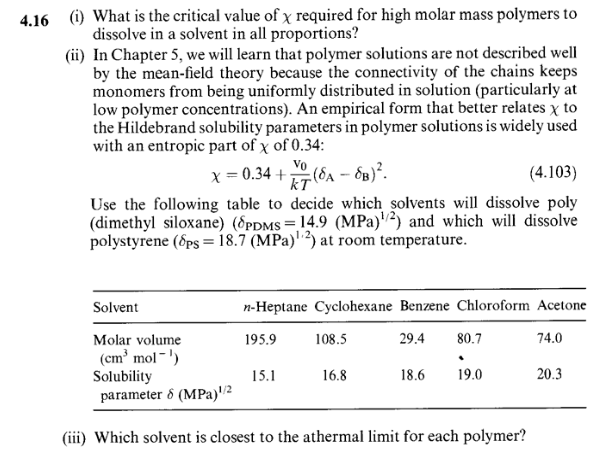
(i) What is the critical value of required for high molar mass polymers to dissolve in a solvent in all proportions? (ii) In Chapter 5 , we will learn that polymer solutions are not described well by the mean-field theory because the connectivity of the chains keeps monomers from being uniformly distributed in solution (particularly at low polymer concentrations). An empirical form that better relates to the Hildebrand solubility parameters in polymer solutions is widely used with an entropic part of of 0.34 : =0.34+kTv0(AB)2. Use the following table to decide which solvents will dissolve poly (dimethyl siloxane) (PDMS=14.9(MPa)1/2) and which will dissolve polystyrene (PS=18.7(MPa)1,2) at room temperature. (iii) Which solvent is closest to the athermal limit for each polymer
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


