Question: For the atom whose energy levels are depicted below, 653 nm light can be absorbed. Describe the energy level transition experienced by an electron when
For the atom whose energy levels are depicted below, 653 nm light can be absorbed. Describe the energy level transition experienced by an electron when this happens.
What is the initial energy level and the the final energy level? What is the equation being used and the steps?
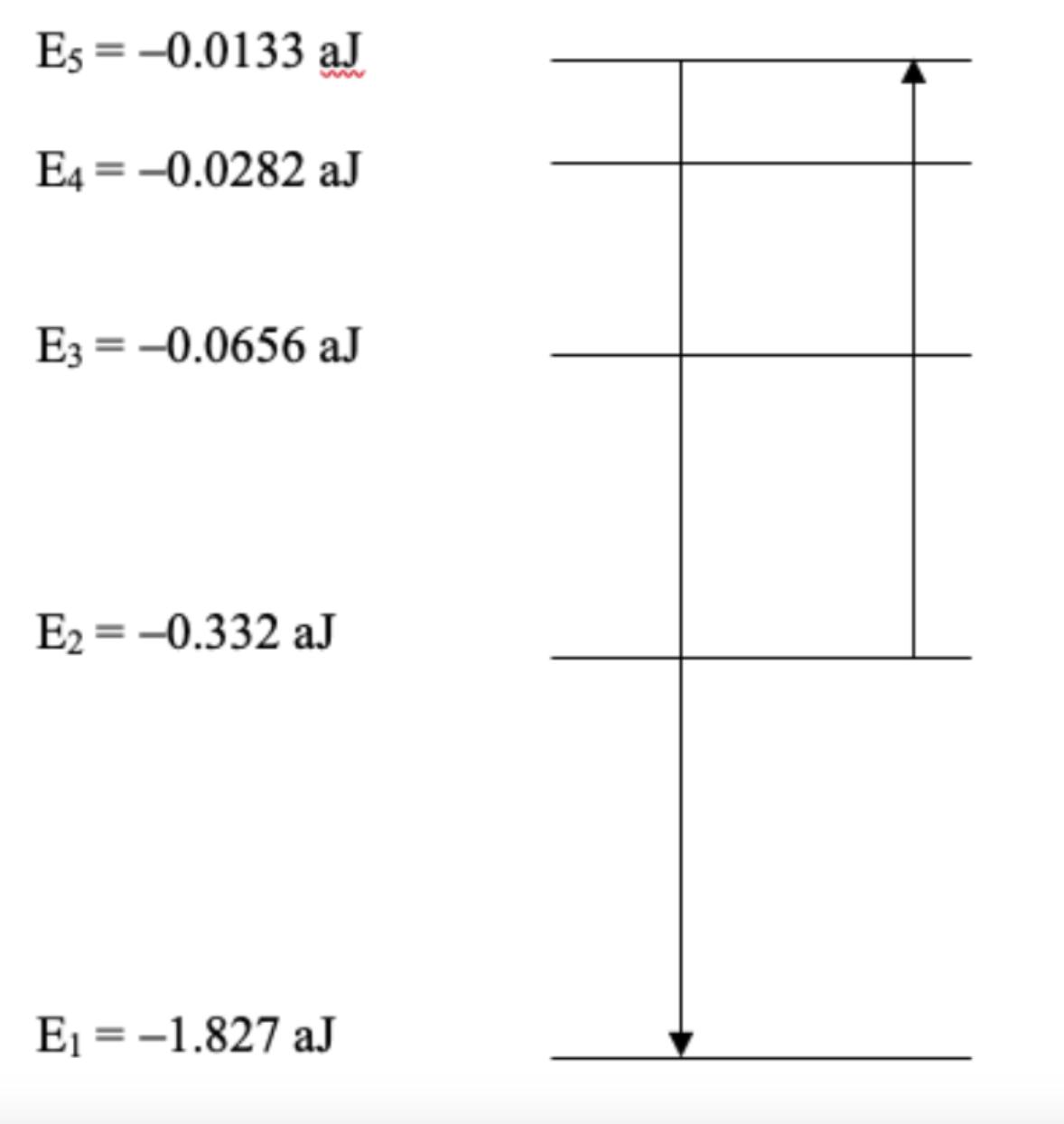
Es=-0.0133 aJ E4 = -0.0282 aJ E3 = -0.0656 aJ E = -0.332 aJ E = -1.827 aJ
Step by Step Solution
There are 3 Steps involved in it
To determine the energy level transition when 653 nm light is absorbed we can use the energywaveleng... View full answer

Get step-by-step solutions from verified subject matter experts


