Question: For the below process, provide linear mass balance (equations and solved split fractions only - do not solve for flow rates) to describe each process
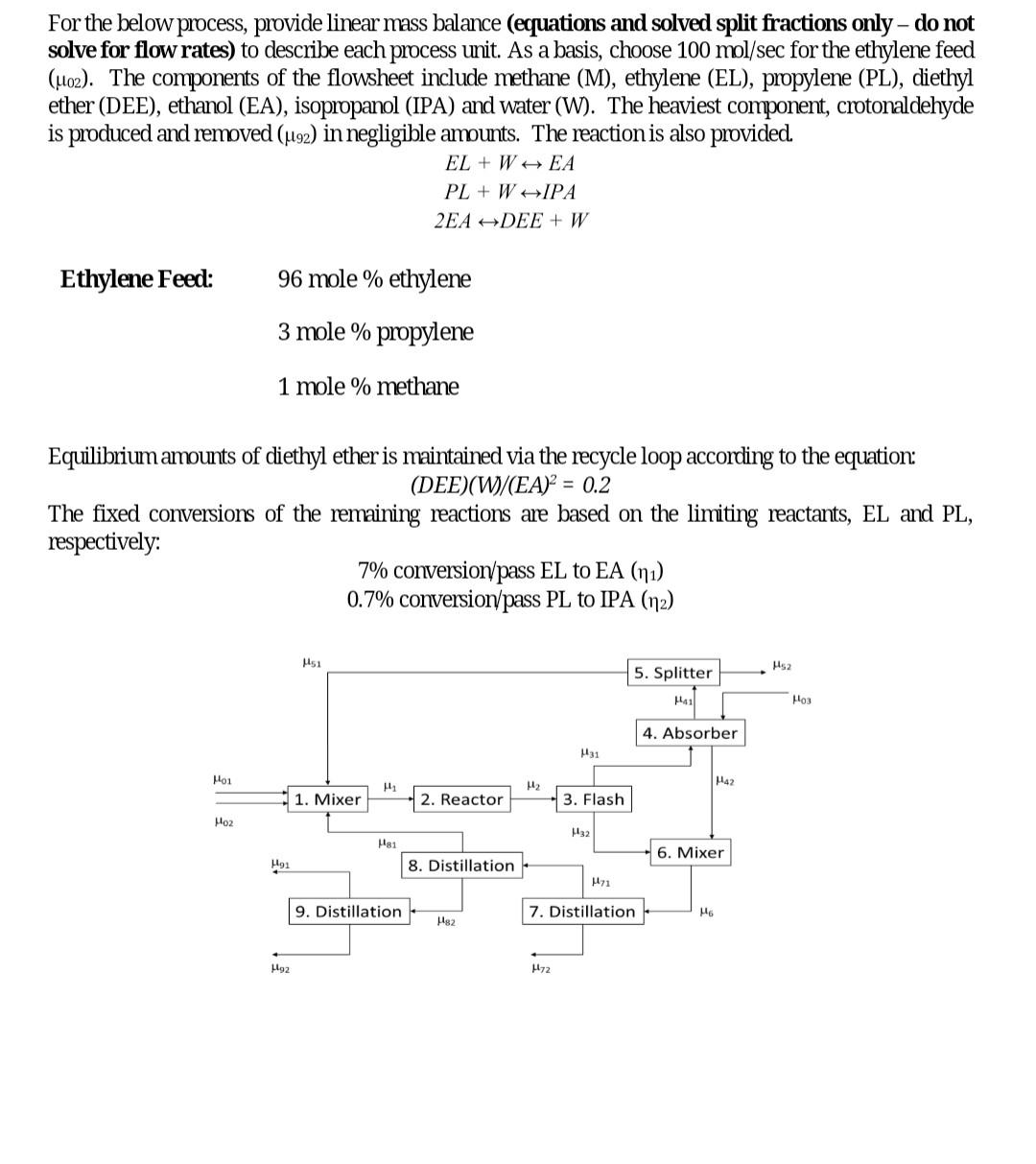
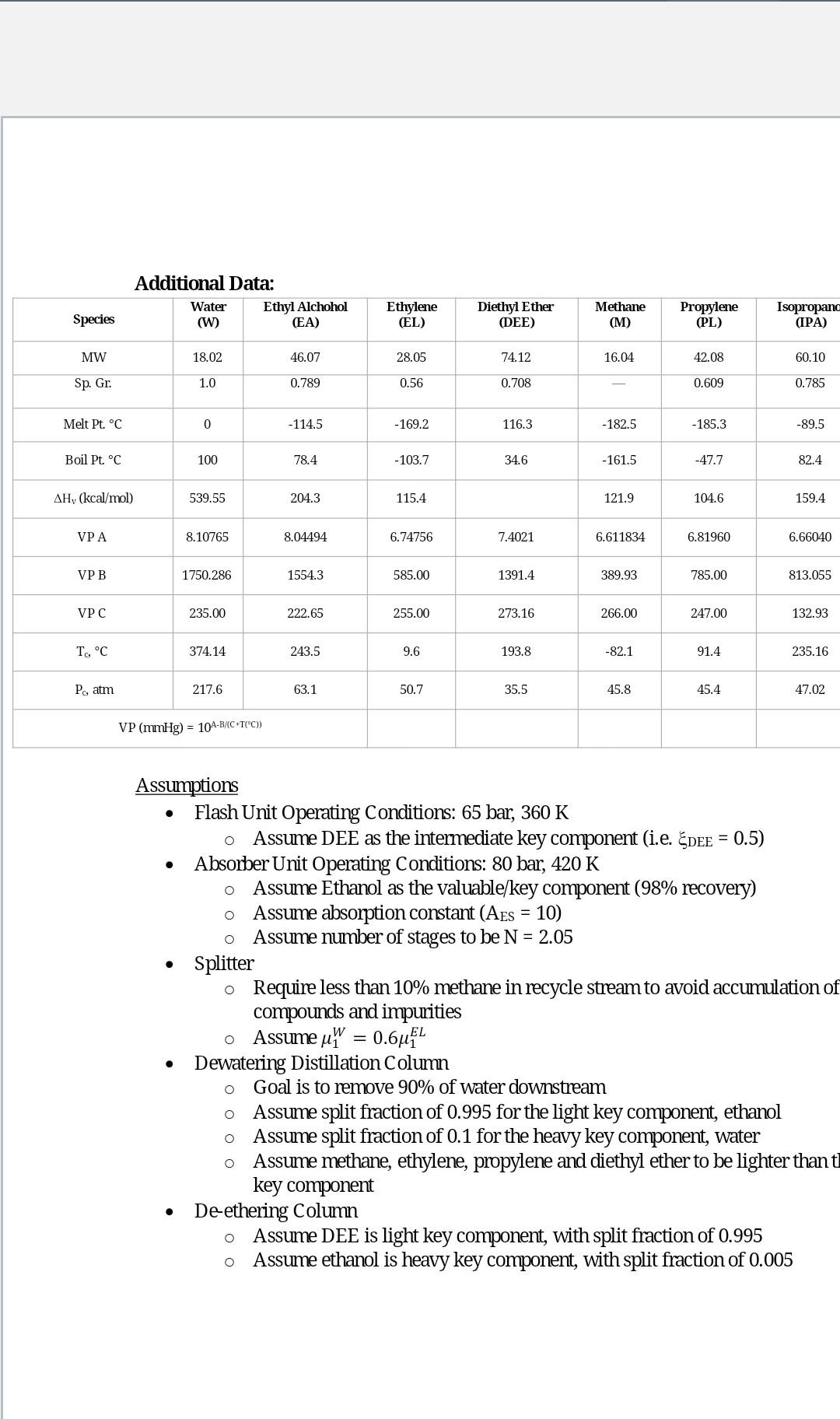
For the below process, provide linear mass balance (equations and solved split fractions only - do not solve for flow rates) to describe each process unit. As a basis, choose 100mol/sec for the ethylene feed (02). The components of the flowsheet include methane (M), ethylene (EL), propylene (PL), diethyl ether (DEE), ethanol (EA), isopropanol (IPA) and water (W). The heaviest component, crotonaldehyde is produced and removed (92) in negligible amounts. The reaction is also provided. EL+WEAPL+WIPA2EADEE+W Equilibrium amounts of diethyl ether is maintained via the recycle loop according to the equation: (DEE)(W)/(EA)2=0.2 The fixed conversions of the remaining reactions are based on the limiting reactants, EL and PL, respectively: Assumptions - Flash Unit Operating Conditions: 65bar,360K - Assume DEE as the intermediate key component (i.e. DEE=0.5 ) - Absorber Unit Operating Conditions: 80bar,420K Assume Ethanol as the valuable/key component (98\% recovery) Assume absorption constant (AES=10) Assume number of stages to be N=2.05 - Splitter Require less than 10% methane in recycle stream to avoid accumulation of compounds and impurities - Assume 1W=0.61EL - Dewatering Distillation Column - Goal is to remove 90% of water downstream Assume split fraction of 0.995 for the light key component, ethanol Assume split fraction of 0.1 for the heavy key component, water Assume methane, ethylene, propylene and diethyl ether to be lighter than key component - De-ethering Column Assume DEE is light key component, with split fraction of 0.995 Assume ethanol is heavy key component, with split fraction of 0.005
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


