Question: For the flash operation, feed entering = 100 k mol/hour with acetone composition of za = .4 and flashed t = 69 C. what is
For the flash operation, feed entering = 100 k mol/hour with acetone composition of za = .4 and flashed t = 69 C.
what is the exiting vapor composition for ya and yb?
what is the existing flow rate? v = ? in kmol/hour
estimate the value of Ka?
split ratio of acetone> what does this value indicate about the quality of separation?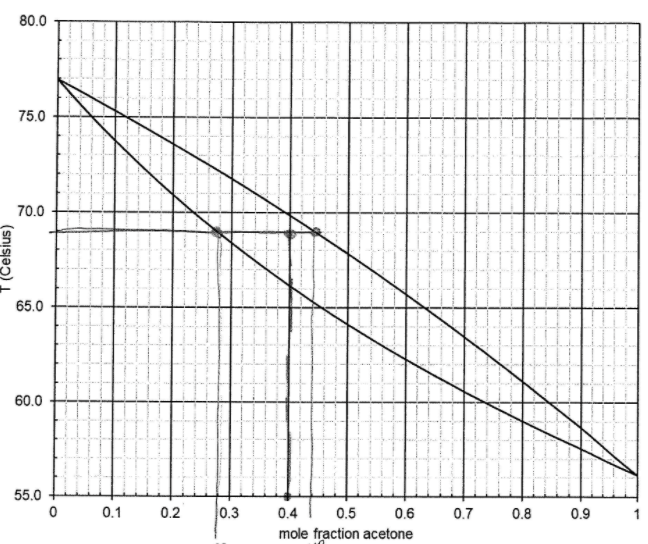
80.0 75.0 70.0 (Celsius) 65.0 60.0 55.0 O 0.1 0.2 0.3 0.4 0.7 0.8 0.9 1 0.5 0.6 mole fraction acetone
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


