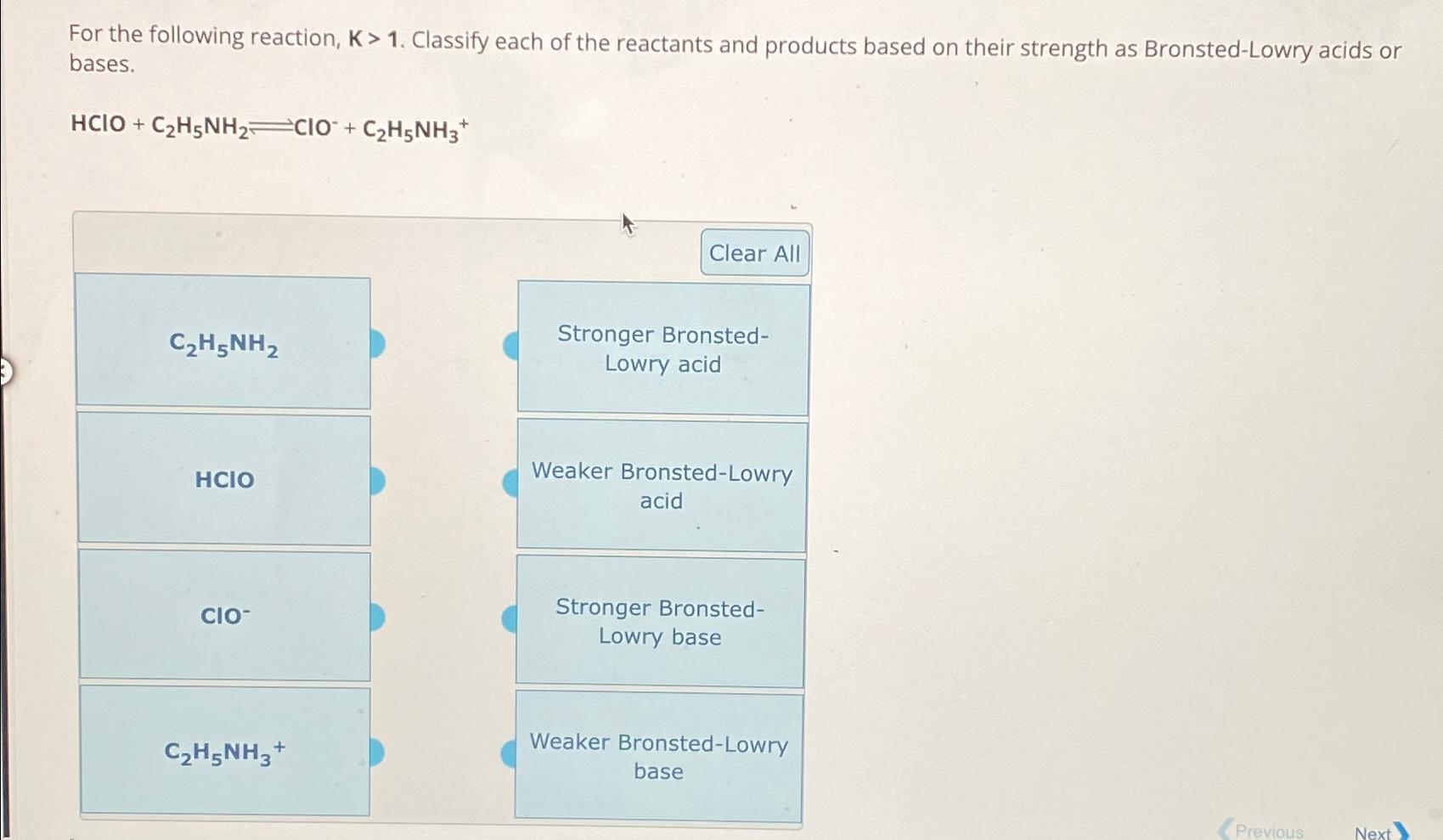
Question: For the following reaction, K > 1 . Classify each of the reactants and products based on their strength as Bronsted - Lowry acids or
For the following reaction, Classify each of the reactants and products based on their strength as BronstedLowry acids or bases.
HClO
tableClear AlltableStronger BronstedLowry acidtableWeaker BronstedLowryacidtableStronger BronstedLowry basetableWeaker BronstedLowrybase
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


