Question: For the galvanic cell below: ( i ) ( 5 % ) Write the 1 2 reactions that occur at cathode and and at anode,
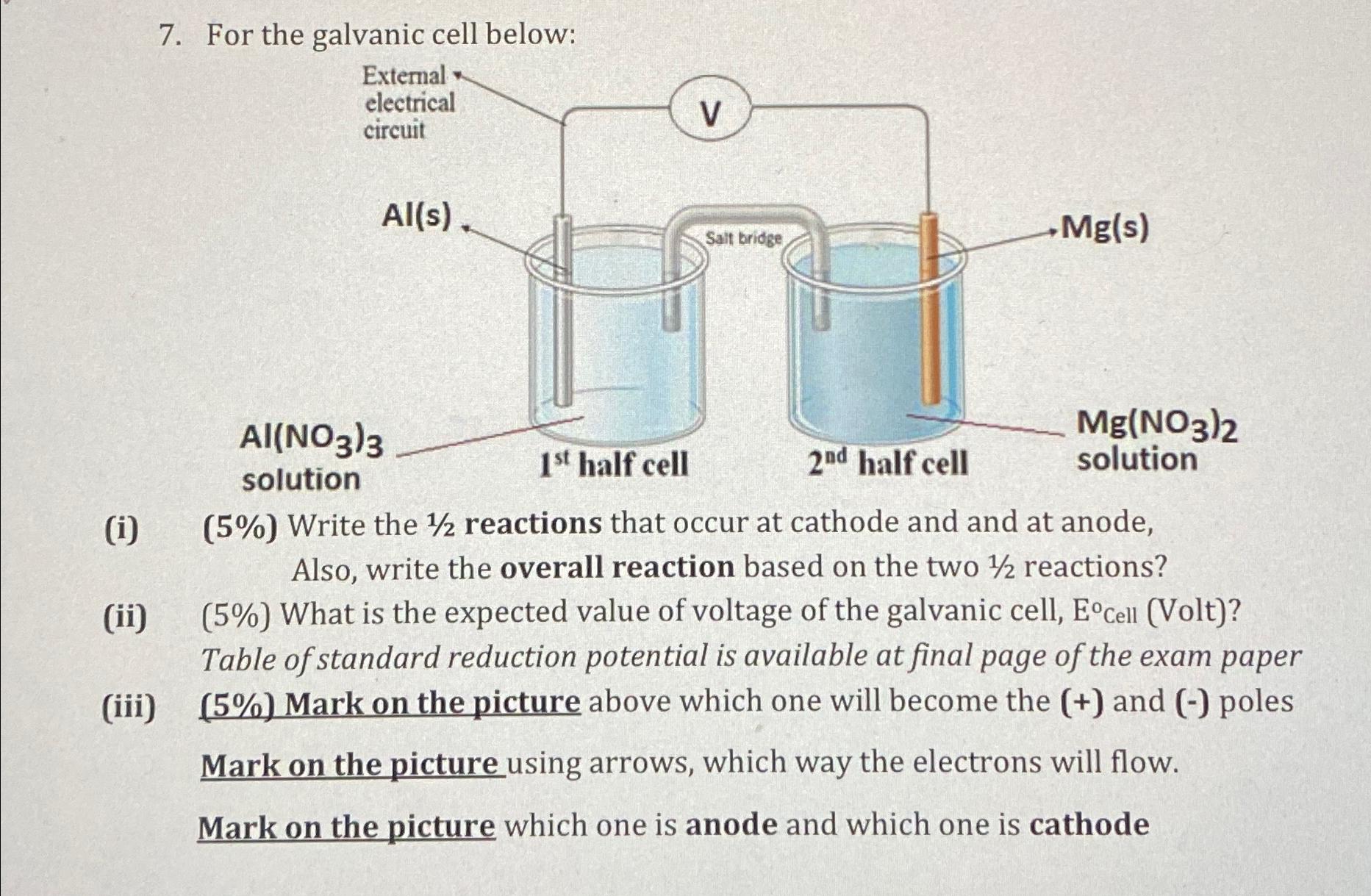
For the galvanic cell below:
i Write the reactions that occur at cathode and and at anode, Also, write the overall reaction based on the two reactions?
ii What is the expected value of voltage of the galvanic cell, Cell Volt Table of standard reduction potential is available at final page of the exam paper
iii Mark on the picture above which one will become the and poles Mark on the picture using arrows, which way the electrons will flow.
Mark on the picture which one is anode and which one is cathode
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


